Answered step by step
Verified Expert Solution
Question
1 Approved Answer
10 mol/s of gas flow through a turbine. Find the change that the gas experiences: The gas is steam, with an inlet temperature and pressure
10 mol/s of gas flow through a turbine. Find the change that the gas experiences:
The gas is steam, with an inlet temperature and pressure T=873.15K and P=10bar,
and an outlet temperature and pressure T=673.15K and P=1bar. Use the steam tables, what is  ?
?
Please answer in as much detail as possible.
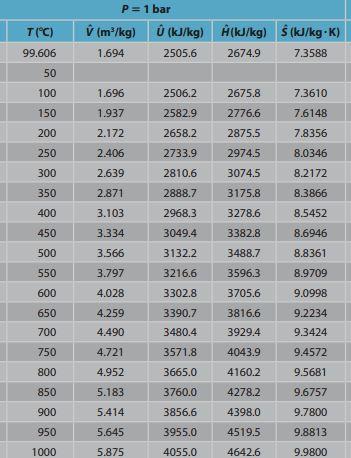
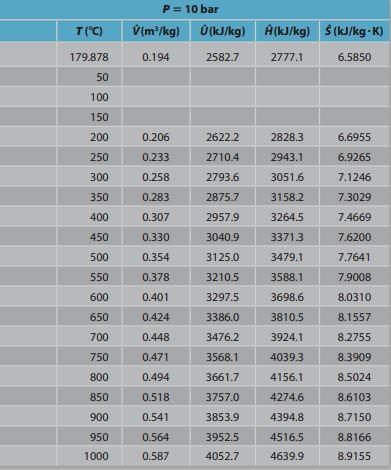
out Steam turbine W out T("C) 99.606 P= 1 bar (m /kg) (kJ/kg) (kJ/kg) (kJ/kg) (kJ/kg-K) ) 1.694 2505.6 2674.9 7.3588 50 100 2506.2 2675.8 7.3610 1.696 1.937 150 2582.9 2776.6 7.6148 200 2.172 2658.2 2875.5 7.8356 250 2.406 2733.9 2974.5 8.0346 300 2.639 2810.6 3074.5 8.2172 350 2.871 2888.7 3175.8 8.3866 400 3.103 2968.3 3278.6 8.5452 450 3.334 3049.4 3382.8 8.6946 500 3566 3132.2 3488.7 8.8361 550 3.797 3216.6 3596.3 8.9709 9.0998 4.028 3302.8 3705.6 600 650 3816.6 9.2234 4.259 4.490 3390.7 3480.4 700 3929.4 9.3424 4.721 3571.8 4043.9 9.4572 750 800 4.952 3665.0 4160.2 9.5681 850 5.183 3760.0 4278.2 9.6757 900 5.414 3856.6 4398.0 9.7800 950 3955.0 4519.5 9.8813 5.645 5.875 1000 4055.0 4642.6 9.9800 T(C) P = 10 bar (m/kg) 0(kJ/kg) 0.194 2582.7 A(kJ/kg) $ (kJ/kg-K) 2777.1 6.5850 179.878 50 100 150 200 0.206 2622.2 2828.3 6.6955 250 0.233 2710.4 2943.1 6.9265 300 0.258 2793.6 3051.6 7.1246 350 0.283 2875.7 3158.2 7.3029 400 0.307 2957.9 3264.5 7.4669 450 0.330 3040.9 7.6200 33713 3479.1 500 3125.0 7.7641 0.354 0.378 550 3210.5 3588.1 7.9008 600 0.401 3297.5 3698.6 8.0310 650 0.424 3386.0 3810.5 8.1557 700 0.448 3476.2 3924.1 8.2755 750 0.471 3568.1 4039.3 8.3909 800 0.494 3661.7 4156.1 8.5024 850 0.518 3757.0 4274.6 8.6103 900 0.541 3853.9 4394.8 8.7150 950 3952.5 4516.5 8.8166 0.564 0.587 1000 4052.7 4639.9 8.9155Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


