Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Enthalpy of Solution Using the value of CP , cal ( - 7 0 . 0 2 5 kg ) calculate the enthalpy of solution
Enthalpy of Solution
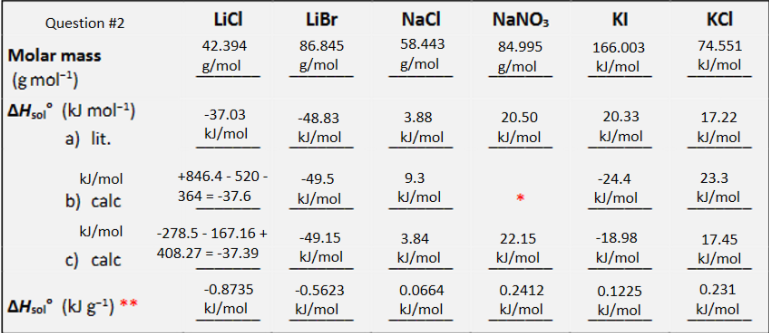
Using the value of CPcal kg calculate the enthalpy of solution in J g for your unknown salt. Identify the salt by comparison to your tabulated values attached pictureComment on the discrepancies between the experimental and literature value of deltaHsol for your salt.
Mass of Clean Dry Calorimeter: g
Mass of Calorimeter water: g
Mass of Calorimeter water salt: g
Initial Calorimeter Temperature Ti degrees celsius
Final Calorimeter Temperature Tf degrees celsius
The cation is lithium and the salt can be:
LiF, Ukj mol
LiCl, Ukj mol
LiBr, Ukj mol
LiI Lithium Iodide Ukj mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


