Question
10. The bond type and molecular polarity of HSe are Bond Type Polarity of Molecule polar nonpolar polar (A) (B) (C) nonpolar (D) nonpolar
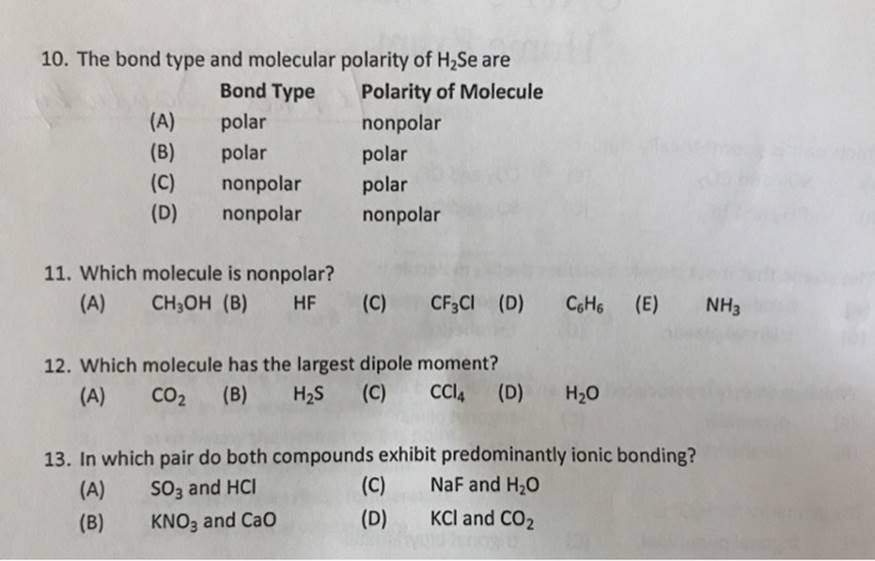
10. The bond type and molecular polarity of HSe are Bond Type Polarity of Molecule polar nonpolar polar (A) (B) (C) nonpolar (D) nonpolar 11. Which molecule is nonpolar? (A) polar polar nonpolar CHOH (B) HF (C) CF3Cl (D) C6H6 (E) 12. Which molecule has the largest dipole moment? (A) CO (B) HS (C) CCl4 (D) HO 13. In which pair do both compounds exhibit predominantly ionic bonding? (A) SO3 and HCI NaF and HO (B) KNO3 and CaO KCI and CO mogu (C) (D) NH3
Step by Step Solution
3.53 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below The correct answer is option b polarpol...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



