Answered step by step
Verified Expert Solution
Question
1 Approved Answer
100 kmol/h of a feed (z) composed of 25 mol% n-butane, 40 mol% n-pentane, and 35 mol% n-hexane is flashed. If 80% (by moles) of
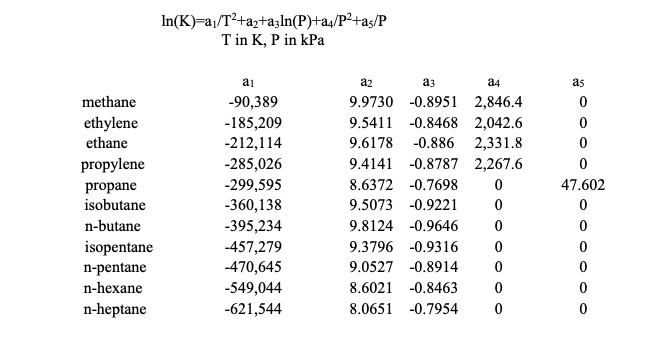
100 kmol/h of a feed (z) composed of 25 mol% n-butane, 40 mol% n-pentane, and 35 mol% n-hexane is flashed. If 80% (by moles) of the n-hexane is in the liquid at 400 K, what is the pressure (P) and the liquid (xi) and vapor (yi) compositions? Obtain K-values from the above table. (Hint: Calculate bubble point and dew point pressures first to get an estimate for an initial input value for equilibrium pressure in the flash calculations.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


