Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The K, for hydrofluoric acid is 7.2 x 104. This means that HF is Save Question 2 (2 points) The stronger the acid, the
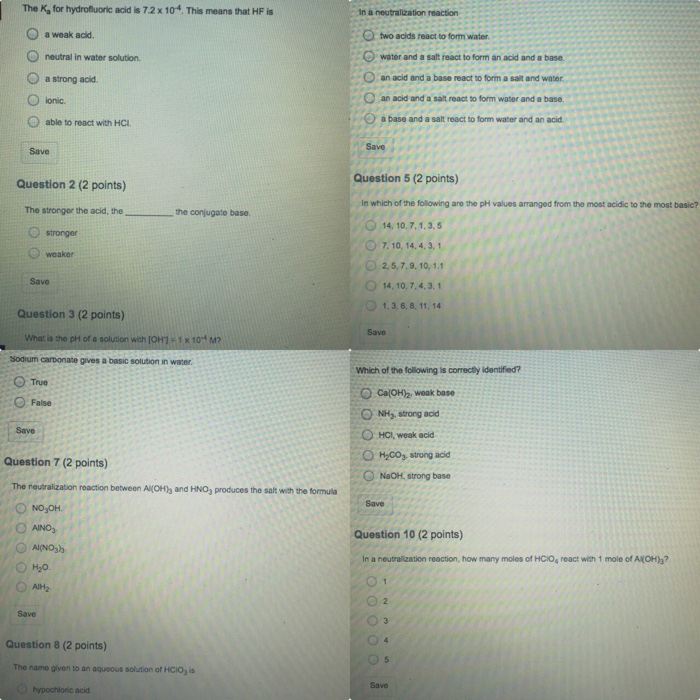
The K, for hydrofluoric acid is 7.2 x 104. This means that HF is Save Question 2 (2 points) The stronger the acid, the stronger weaker a weak acid. neutral in water solution. a strong acid. lonic. able to react with HCL. Save 0 0 0 Question 3 (2 points) What is the pH of a solution with [OH]-1 x 10 M? Sodium carbonate gives a basic solution in water. Save True False the conjugate base. Question 7 (2 points) The neutralization reaction between Al(OH)3 and HNO3 produces the salt with the formula NOOH. AINO AI(NO) HO. AIH. Save Question 8 (2 points) The name given to an aqueous solution of HCIO, is hypochloric acid In a neutralization reaction Otwo acids react to form water. water and a salt react to form an acid and a base. an acid and a base react to form a salt and water. Oan acid and a salt react to form water and a base. a base and a salt react to form water and an acid. Save Question 5 (2 points) In which of the following are the pH values arranged from the most acidic to the most basic? 14, 10, 7, 1, 3, 5 7, 10, 14, 4, 3, 1 2,5, 7, 9, 10, 1.1 14, 10, 7, 4, 3, 1 1, 3, 6, 8, 11, 14 Save Which of the following is correctly identified? Ca(OH), weak base ONH, strong acid OHCI, weak acid OHCO3, strong acid O NaOH, strong base Save Question 10 (2 points) In a neutralization reaction, how many moles of HCIO, react with 1 mole of Al(OH)3? 2 3 Save
Step by Step Solution
★★★★★
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below 1 The K for hydrofluoric acid is 72 x 104 This means that HF is a weak acid The K for an acid is a measure of its acidity It is the equilib...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


