Answered step by step
Verified Expert Solution
Question
1 Approved Answer
15. For a first-order reaction, the hal-life is constant: it depends ony on the rate constant k and not The hali-life of a reaction, t1/2,
15. 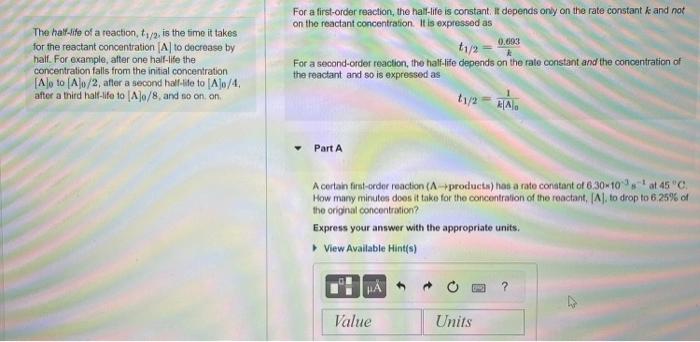
For a first-order reaction, the hal-life is constant: it depends ony on the rate constant k and not The hali-life of a reaction, t1/2, is the time it takes on the reactant concentration. If is expressed as for the reactant concentration [A] to decrease by haif. For example, after one half-ife the concentration falls from the initial concentration [A]0 to [A]0/2, afler a second hal-lile to [A]0/4, after a third half-life to [A]0/8, and e0 on. on. Part A t1/2=k[A]01 A certan first-order reaction (Aproducts) has a rate conatant of 6.30m103s1 at 451C : How mary minutes does it take for the concentration of the reactant, [A], to drop to 6.25%6 of the original concentration? Express your answer with the appropriate units 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


