Answered step by step
Verified Expert Solution
Question
1 Approved Answer
15. Since it was first discovered in 1968 , the enzyme superoxide dismutase, SOD, has been found to be one of the most widely dispersed
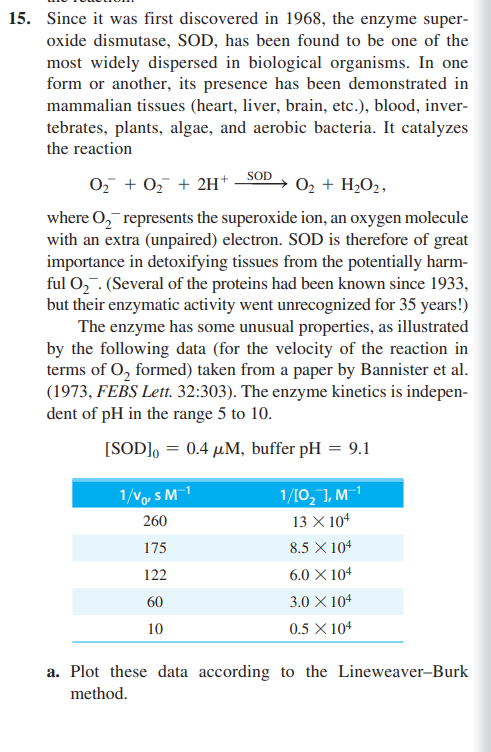
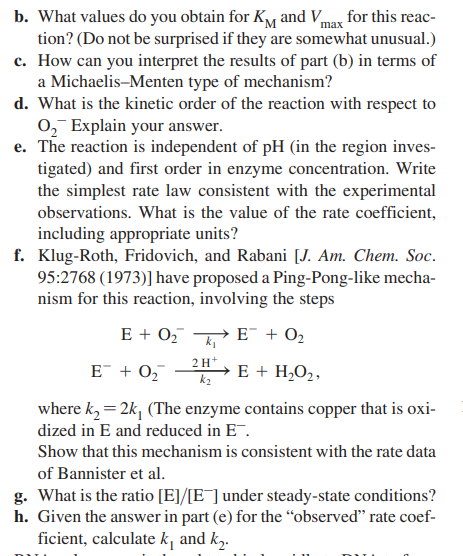 15. Since it was first discovered in 1968 , the enzyme superoxide dismutase, SOD, has been found to be one of the most widely dispersed in biological organisms. In one form or another, its presence has been demonstrated in mammalian tissues (heart, liver, brain, etc.), blood, invertebrates, plants, algae, and aerobic bacteria. It catalyzes the reaction O2+O2+2H+SODO2+H2O2, where O2represents the superoxide ion, an oxygen molecule with an extra (unpaired) electron. SOD is therefore of great importance in detoxifying tissues from the potentially harmful O2. (Several of the proteins had been known since 1933, but their enzymatic activity went unrecognized for 35 years!) The enzyme has some unusual properties, as illustrated by the following data (for the velocity of the reaction in terms of O2 formed) taken from a paper by Bannister et al. (1973, FEBS Lett. 32:303). The enzyme kinetics is independent of pH in the range 5 to 10 . [SOD]0=0.4M, buffer pH=9.1 a. Plot these data according to the Lineweaver-Burk method. b. What values do you obtain for KM and Vmax for this reaction? (Do not be surprised if they are somewhat unusual.) c. How can you interpret the results of part (b) in terms of a Michaelis-Menten type of mechanism? d. What is the kinetic order of the reaction with respect to O2Explain your answer. e. The reaction is independent of pH (in the region investigated) and first order in enzyme concentration. Write the simplest rate law consistent with the experimental observations. What is the value of the rate coefficient, including appropriate units? f. Klug-Roth, Fridovich, and Rabani [J. Am. Chem. Soc. 95:2768 (1973)] have proposed a Ping-Pong-like mechanism for this reaction, involving the steps E+O2k1E+O2E+O2k22H+E+H2O2, where k2=2k1 (The enzyme contains copper that is oxidized in E and reduced in E. Show that this mechanism is consistent with the rate data of Bannister et al. g. What is the ratio [E]/[E]under steady-state conditions? h. Given the answer in part (e) for the "observed" rate coefficient, calculate k1 and k2
15. Since it was first discovered in 1968 , the enzyme superoxide dismutase, SOD, has been found to be one of the most widely dispersed in biological organisms. In one form or another, its presence has been demonstrated in mammalian tissues (heart, liver, brain, etc.), blood, invertebrates, plants, algae, and aerobic bacteria. It catalyzes the reaction O2+O2+2H+SODO2+H2O2, where O2represents the superoxide ion, an oxygen molecule with an extra (unpaired) electron. SOD is therefore of great importance in detoxifying tissues from the potentially harmful O2. (Several of the proteins had been known since 1933, but their enzymatic activity went unrecognized for 35 years!) The enzyme has some unusual properties, as illustrated by the following data (for the velocity of the reaction in terms of O2 formed) taken from a paper by Bannister et al. (1973, FEBS Lett. 32:303). The enzyme kinetics is independent of pH in the range 5 to 10 . [SOD]0=0.4M, buffer pH=9.1 a. Plot these data according to the Lineweaver-Burk method. b. What values do you obtain for KM and Vmax for this reaction? (Do not be surprised if they are somewhat unusual.) c. How can you interpret the results of part (b) in terms of a Michaelis-Menten type of mechanism? d. What is the kinetic order of the reaction with respect to O2Explain your answer. e. The reaction is independent of pH (in the region investigated) and first order in enzyme concentration. Write the simplest rate law consistent with the experimental observations. What is the value of the rate coefficient, including appropriate units? f. Klug-Roth, Fridovich, and Rabani [J. Am. Chem. Soc. 95:2768 (1973)] have proposed a Ping-Pong-like mechanism for this reaction, involving the steps E+O2k1E+O2E+O2k22H+E+H2O2, where k2=2k1 (The enzyme contains copper that is oxidized in E and reduced in E. Show that this mechanism is consistent with the rate data of Bannister et al. g. What is the ratio [E]/[E]under steady-state conditions? h. Given the answer in part (e) for the "observed" rate coefficient, calculate k1 and k2 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


