Answered step by step
Verified Expert Solution
Question
1 Approved Answer
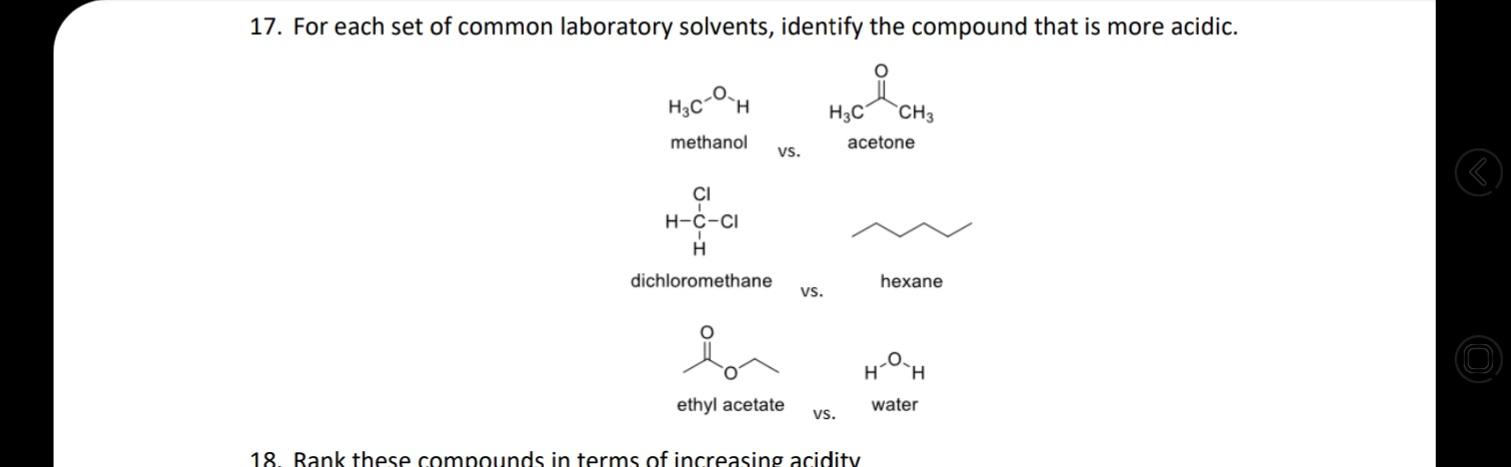
18. Rank these compounds in terms of increasing acidity HNO, H,SO4 HF H,CO3 . NH3 CH4 H2O HCI b. OH NH2 HO . HF
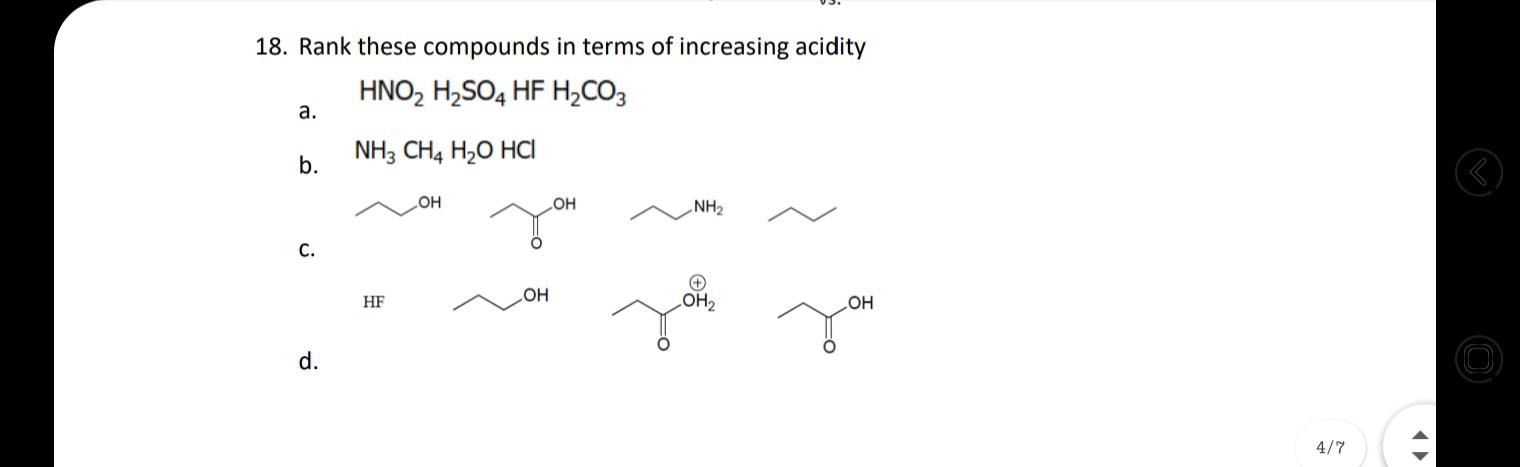
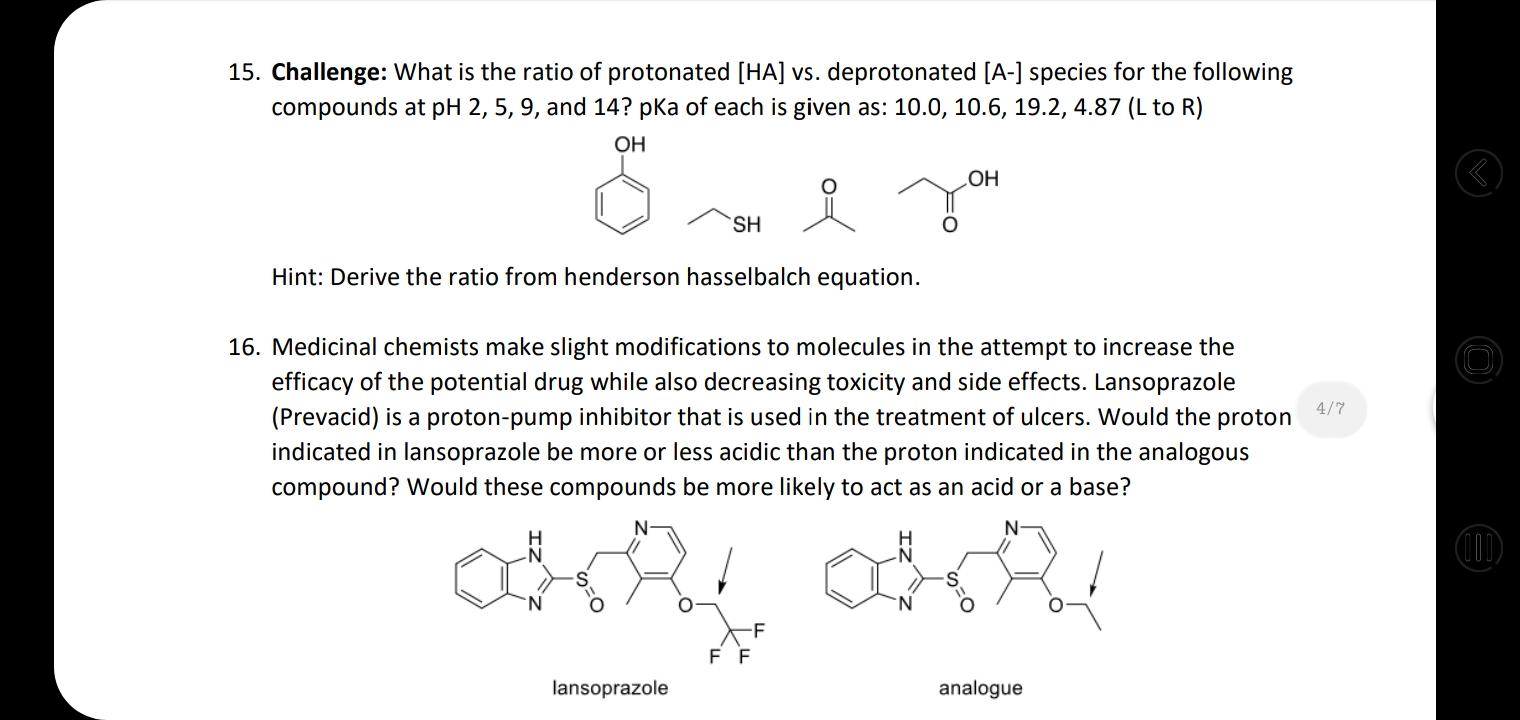
18. Rank these compounds in terms of increasing acidity HNO, H,SO4 HF H,CO3 . NH3 CH4 H2O HCI b. OH NH2 HO . HF LOH2 d. 4/7 17. For each set of common laboratory solvents, identify the compound that is more acidic. H3C `CH3 methanol acetone vs. CI H-C-CI H dichloromethane hexane vs. H ethyl acetate water vs. 18. Rank these compounds in terms of increasing acidity 15. Challenge: What is the ratio of protonated [HA] vs. deprotonated [A-] species for the following compounds at pH 2, 5, 9, and 14? pka of each is given as: 10.0, 10.6, 19.2, 4.87 (L to R) OH HO SH Hint: Derive the ratio from henderson hasselbalch equation. 16. Medicinal chemists make slight modifications to molecules in the attempt to increase the | O efficacy of the potential drug while also decreasing toxicity and side effects. Lansoprazole 4/7 (Prevacid) is a proton-pump inhibitor that is used in the treatment of ulcers. Would the proton indicated in lansoprazole be more or less acidic than the proton indicated in the analogous compound? Would these compounds be more likely to act as an acid or a base? (1) -F F F lansoprazole analogue
Step by Step Solution
★★★★★
3.45 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started