Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1) A 0.25 M solution of a monoprotic weak acid has a percent ionization of 1.50%. Determine the Ka of this weak acid. 2)
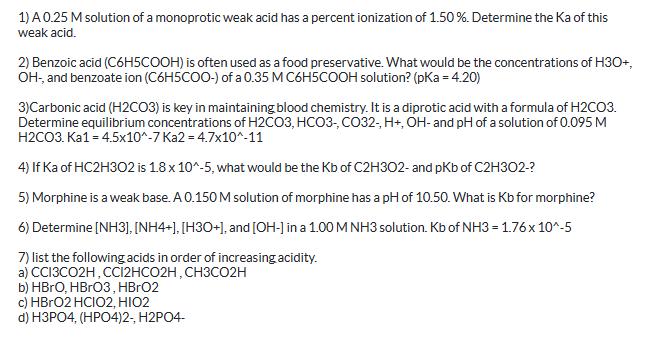
1) A 0.25 M solution of a monoprotic weak acid has a percent ionization of 1.50%. Determine the Ka of this weak acid. 2) Benzoic acid (C6H5COOH) is often used as a food preservative. What would be the concentrations of H3O+, OH-, and benzoate ion (C6H5COO-) of a 0.35 M C6H5COOH solution? (pKa = 4.20) 3)Carbonic acid (H2CO3) is key in maintaining blood chemistry. It is a diprotic acid with a formula of H2CO3. Determine equilibrium concentrations of H2CO3, HCO3-, CO32-, H+, OH- and pH of a solution of 0.095 M H2CO3. Ka1 = 4.5x10^-7 Ka2-4.7x10^-11 4) If Ka of HC2H3O2 is 1.8 x 10^-5, what would be the Kb of C2H302- and pKb of C2H302-? 5) Morphine is a weak base. A 0.150 M solution of morphine has a pH of 10.50. What is Kb for morphine? 6) Determine [NH3], [NH4+], [H3O+], and [OH-] in a 1.00 M NH3 solution. Kb of NH3 = 1.76 x 10^-5 7) list the following acids in order of increasing acidity. a) CC13CO2H, CC12HCO2H, CH3CO2H b) HBrO, HBrO3, HBrO2 c) HBrO2 HCIO2, HIO2 d) H3PO4, (HPO4)2-, H2PO4-
Step by Step Solution
★★★★★
3.33 Rating (144 Votes )
There are 3 Steps involved in it
Step: 1
1 A 025 M solution of a monoprotic weak acid has a percent ionization of 150 To find the Ka ionizati...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


