Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1.Show that the thermodynamic equation of state may be expressed in the equvalent form and for an Adiabatic process of an ideal gas can be
1.Show that the thermodynamic equation of state 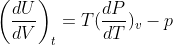 may be expressed in the equvalent form
may be expressed in the equvalent form 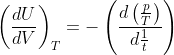
and for an Adiabatic process of an ideal gas can be described by equation pV = const where = Cp/Cv 1.4. Which of the following diagrams most precisely represents such a process in coordinates p-T ? Justify your choice. A) B) C) D) decreasing increasing increasing decreasing concave concave convex convex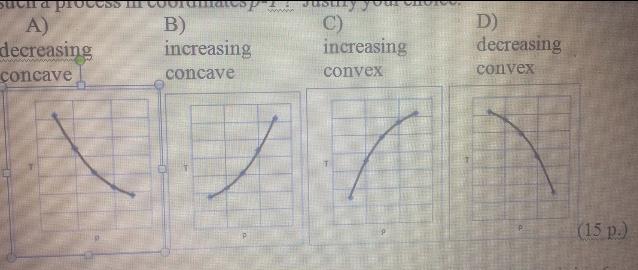
dU dV = dP =TG) d -P dU d (4) (), AP FP T wwww Suva PICUS A) decreasing concave B) increasing concave C) increasing convex D) decreasing convex (15 p.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


