Question
500 mls of a gas is collected over water at 298 K and 101.169 kpa. What is the volume of the dry gas at

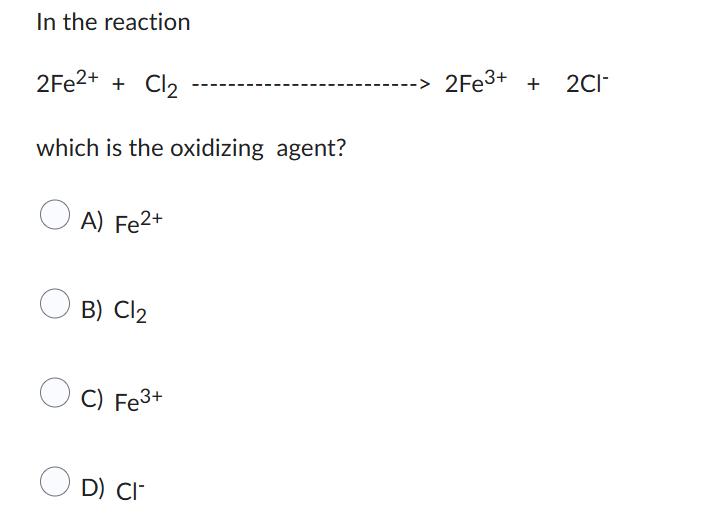
500 mls of a gas is collected over water at 298 K and 101.169 kpa. What is the volume of the dry gas at STP? The vp of water at that T is 3.169 kpa A) 221 mls B) 600 mls C) 350 mls D) 443 mls In the reaction 2Fe2+ + Cl2 which is the oxidizing agent? A) Fe2+ B) Cl C) Fe3+ D) CI- 2Fe3+ + 2C-
Step by Step Solution
3.32 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Intermediate Accounting
Authors: Donald Kieso, Jerry Weygandt, Terry Warfield, Nicola Young,
10th Canadian Edition, Volume 1
978-1118735329, 9781118726327, 1118735323, 1118726324, 978-0176509736
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



