Question
Given the following table of standard reduction potentials for a hypothetical element X under acidic solution: Cell Notation E (V) XO3/XO2 XO2/HXO2 XO:/XO HXO2/XO
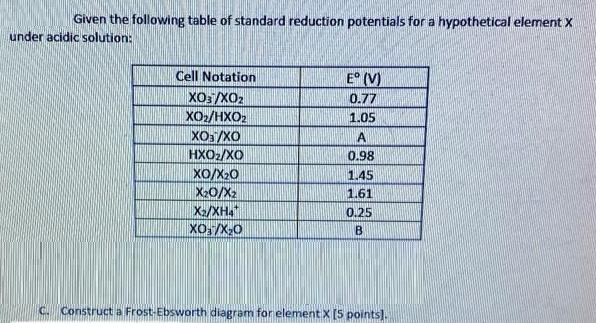
Given the following table of standard reduction potentials for a hypothetical element X under acidic solution: Cell Notation E (V) XO3/XO2 XO2/HXO2 XO:/XO HXO2/XO 0.77 1.05 A 0.98 Xo/X20 X20/X2 1.45 1.61 X2/XHa" XO:/X-0 0.25 C. Construct a Frost-Ebsworth diagram for element X[5 points).
Step by Step Solution
3.48 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



