Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. [20 pts] Consider the following reactions. A2+BA2BA2+2B2AB It is known that reaction (1) releases more Gibb's free energy. For these reactions, following fuel cell
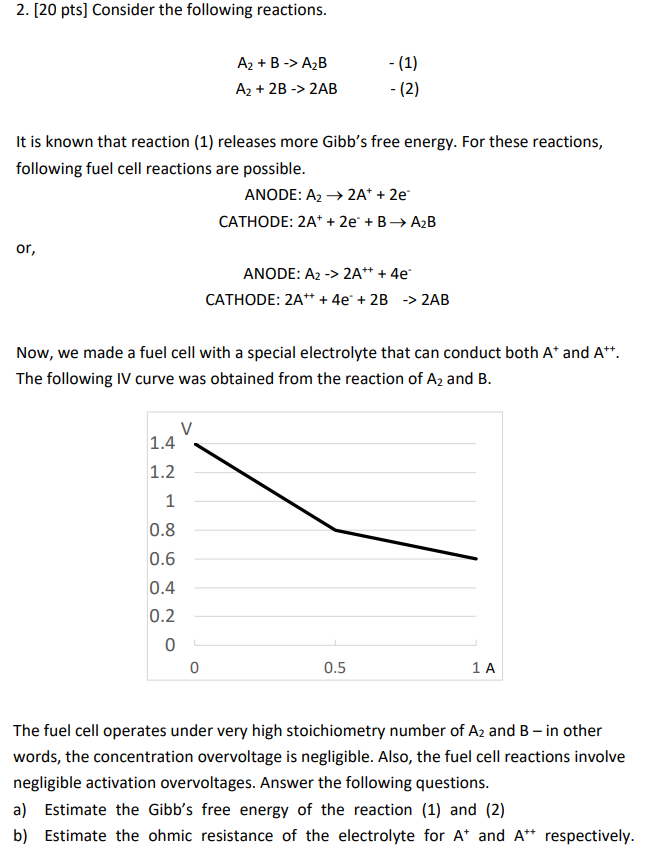 2. [20 pts] Consider the following reactions. A2+BA2BA2+2B2AB It is known that reaction (1) releases more Gibb's free energy. For these reactions, following fuel cell reactions are possible. ANODE: A22A++2e CATHODE: 2A++2e+BA2B or, ANODE: A22A+++4e CATHODE: 2A+++4e+2B2AB Now, we made a fuel cell with a special electrolyte that can conduct both A+and A++. The following IV curve was obtained from the reaction of A2 and B. The fuel cell operates under very high stoichiometry number of A2 and B - in other words, the concentration overvoltage is negligible. Also, the fuel cell reactions involve negligible activation overvoltages. Answer the following questions. a) Estimate the Gibb's free energy of the reaction (1) and (2) b) Estimate the ohmic resistance of the electrolyte for A+and A++respectively
2. [20 pts] Consider the following reactions. A2+BA2BA2+2B2AB It is known that reaction (1) releases more Gibb's free energy. For these reactions, following fuel cell reactions are possible. ANODE: A22A++2e CATHODE: 2A++2e+BA2B or, ANODE: A22A+++4e CATHODE: 2A+++4e+2B2AB Now, we made a fuel cell with a special electrolyte that can conduct both A+and A++. The following IV curve was obtained from the reaction of A2 and B. The fuel cell operates under very high stoichiometry number of A2 and B - in other words, the concentration overvoltage is negligible. Also, the fuel cell reactions involve negligible activation overvoltages. Answer the following questions. a) Estimate the Gibb's free energy of the reaction (1) and (2) b) Estimate the ohmic resistance of the electrolyte for A+and A++respectively Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


