
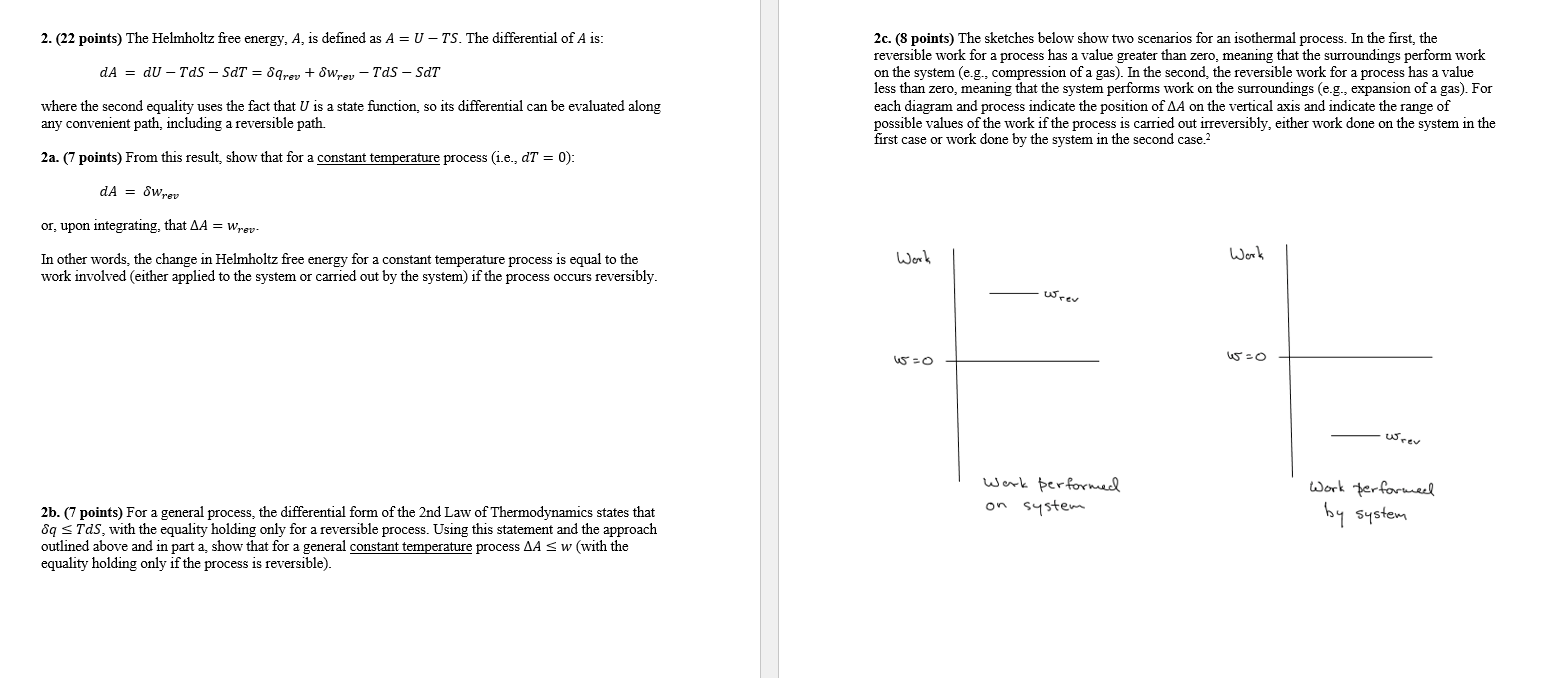
2. (22 points) The Helmholtz free energy, A, is defined as A = U TS. The differential of A is: dA = dU Tds - SdT = Sqrev + Swrev Tds - SDT where the second equality uses the fact that U is a state function, so its differential can be evaluated along any convenient path, including a reversible path. 2a. (7 points) From this result, show that for a constant temperature process (i.e., dt = 0): dA = Wrev or, upon integrating, that AA = Wrev: In other words, the change in Helmholtz free energy for a constant temperature process is equal to the work involved (either applied to the system or carried out by the system) if the process occurs reversibly. 2b. (7 points) For a general process, the differential form of the 2nd Law of Thermodynamics states that 89 s Tds, with the equality holding only for a reversible process. Using this statement and the approach outlined above and in part a, show that for a general constant temperature process AA sw (with the equality holding only if the process is reversible). 2c. (8 points) The sketches below show two scenarios for an isothermal process. In the first, the reversible work for a process has a value greater than zero, meaning that the surroundings perform work on the system (e.g., compression of a gas). In the second, the reversible work for a process has a value less than zero, meaning that the system performs work on the surroundings (e.g., expansion of a gas). For each diagram and process indicate the position of AA on the vertical axis and indicate the range of possible values of the work if the process is carried out irreversibly, either work done on the system in the first case or work done by the system in the second case.? Work Work useo 15-O wrev Werk performed on system Work performed by system 2. (22 points) The Helmholtz free energy, A, is defined as A = U - TS. The differential of A is: dA = dU TAS SdT = Sqrey + wrey Tds SdT 2c. (8 points) The sketches below show two scenarios for an isothermal process. In the first, the reversible work for a process has a value greater than zero, meaning that the surroundings perform work on the system (e.g., compression of a gas). In the second the reversible work for a process has a value less than zero, meaning that the system performs work on the surroundings (eg., expansion of a gas). For each diagram and process indicate the position of AA on the vertical axis and indicate the range of possible values of the work if the process is carried out irreversibly, either work done on the system in the first case or work done by the system in the second case.? where the second equality uses the fact that U is a state function, so its differential can be evaluated along any convenient path, including a reversible path. 2a. (7 points) From this result show that for a constant temperature process (i.e., dT = 0): dA = SWrev Or, upon integrating, that AA = Wrev- Work Work In other words, the change in Helmholtz free energy for a constant temperature process is equal to the work involved (either applied to the system or carried out by the system) if the process occurs reversibly. cev 15-0 5-O wrev Work performed on system Work performed by system 2b. (7 points) For a general process, the differential form of the 2nd Law of Thermodynamics states that 89 5 Tds, with the equality holding only for a reversible process. Using this statement and the approach outlined above and in part a, show that for a general constant temperature process AA S w (with the equality holding only if the process is reversible). 2. (22 points) The Helmholtz free energy, A, is defined as A = U TS. The differential of A is: dA = dU Tds - SdT = Sqrev + Swrev Tds - SDT where the second equality uses the fact that U is a state function, so its differential can be evaluated along any convenient path, including a reversible path. 2a. (7 points) From this result, show that for a constant temperature process (i.e., dt = 0): dA = Wrev or, upon integrating, that AA = Wrev: In other words, the change in Helmholtz free energy for a constant temperature process is equal to the work involved (either applied to the system or carried out by the system) if the process occurs reversibly. 2b. (7 points) For a general process, the differential form of the 2nd Law of Thermodynamics states that 89 s Tds, with the equality holding only for a reversible process. Using this statement and the approach outlined above and in part a, show that for a general constant temperature process AA sw (with the equality holding only if the process is reversible). 2c. (8 points) The sketches below show two scenarios for an isothermal process. In the first, the reversible work for a process has a value greater than zero, meaning that the surroundings perform work on the system (e.g., compression of a gas). In the second, the reversible work for a process has a value less than zero, meaning that the system performs work on the surroundings (e.g., expansion of a gas). For each diagram and process indicate the position of AA on the vertical axis and indicate the range of possible values of the work if the process is carried out irreversibly, either work done on the system in the first case or work done by the system in the second case.? Work Work useo 15-O wrev Werk performed on system Work performed by system 2. (22 points) The Helmholtz free energy, A, is defined as A = U - TS. The differential of A is: dA = dU TAS SdT = Sqrey + wrey Tds SdT 2c. (8 points) The sketches below show two scenarios for an isothermal process. In the first, the reversible work for a process has a value greater than zero, meaning that the surroundings perform work on the system (e.g., compression of a gas). In the second the reversible work for a process has a value less than zero, meaning that the system performs work on the surroundings (eg., expansion of a gas). For each diagram and process indicate the position of AA on the vertical axis and indicate the range of possible values of the work if the process is carried out irreversibly, either work done on the system in the first case or work done by the system in the second case.? where the second equality uses the fact that U is a state function, so its differential can be evaluated along any convenient path, including a reversible path. 2a. (7 points) From this result show that for a constant temperature process (i.e., dT = 0): dA = SWrev Or, upon integrating, that AA = Wrev- Work Work In other words, the change in Helmholtz free energy for a constant temperature process is equal to the work involved (either applied to the system or carried out by the system) if the process occurs reversibly. cev 15-0 5-O wrev Work performed on system Work performed by system 2b. (7 points) For a general process, the differential form of the 2nd Law of Thermodynamics states that 89 5 Tds, with the equality holding only for a reversible process. Using this statement and the approach outlined above and in part a, show that for a general constant temperature process AA S w (with the equality holding only if the process is reversible)








