Answered step by step
Verified Expert Solution
Question
1 Approved Answer
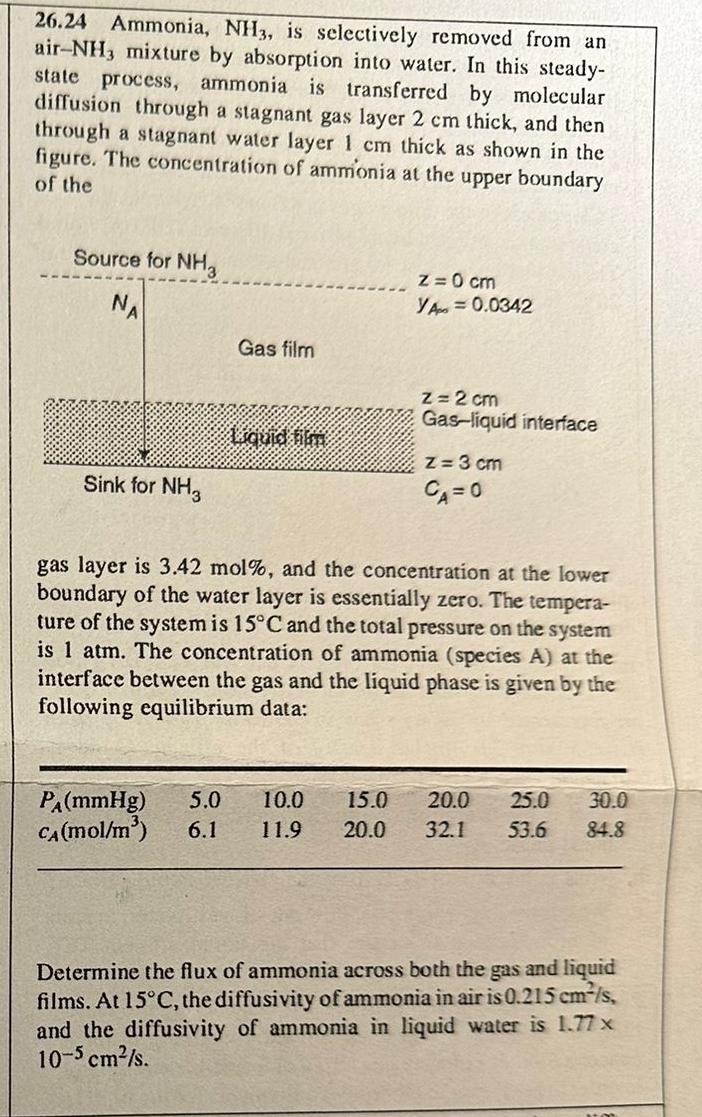
2 6 . 2 4 Ammonia, N H 3 , is selectively removed from an air - N H 3 mixture by absorption into water.
Ammonia, is selectively removed from an air mixture by absorption into water. In this steadystate process, ammonia is transferred by molecular diffusion through a stagnant gas layer thick, and then through a stagnant water layer thick as shown in the figure. The concentration of ammionia at the upper boundary of the
gas layer is mol and the concentration at the lower boundary of the water layer is essentially zero. The temperature of the system is and the total pressure on the system is atm. The concentration of ammonia species A at the interface between the gas and the liquid phase is given by the following equilibrium data:
table
Determine the flux of ammonia across both the gas and liquid films. At the diffusivity of ammonia in air is and the diffusivity of ammonia in liquid water is
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


