Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. (7 pt) The potential energy between two Helium (He) atoms is represented by the following equation as a result of the van der
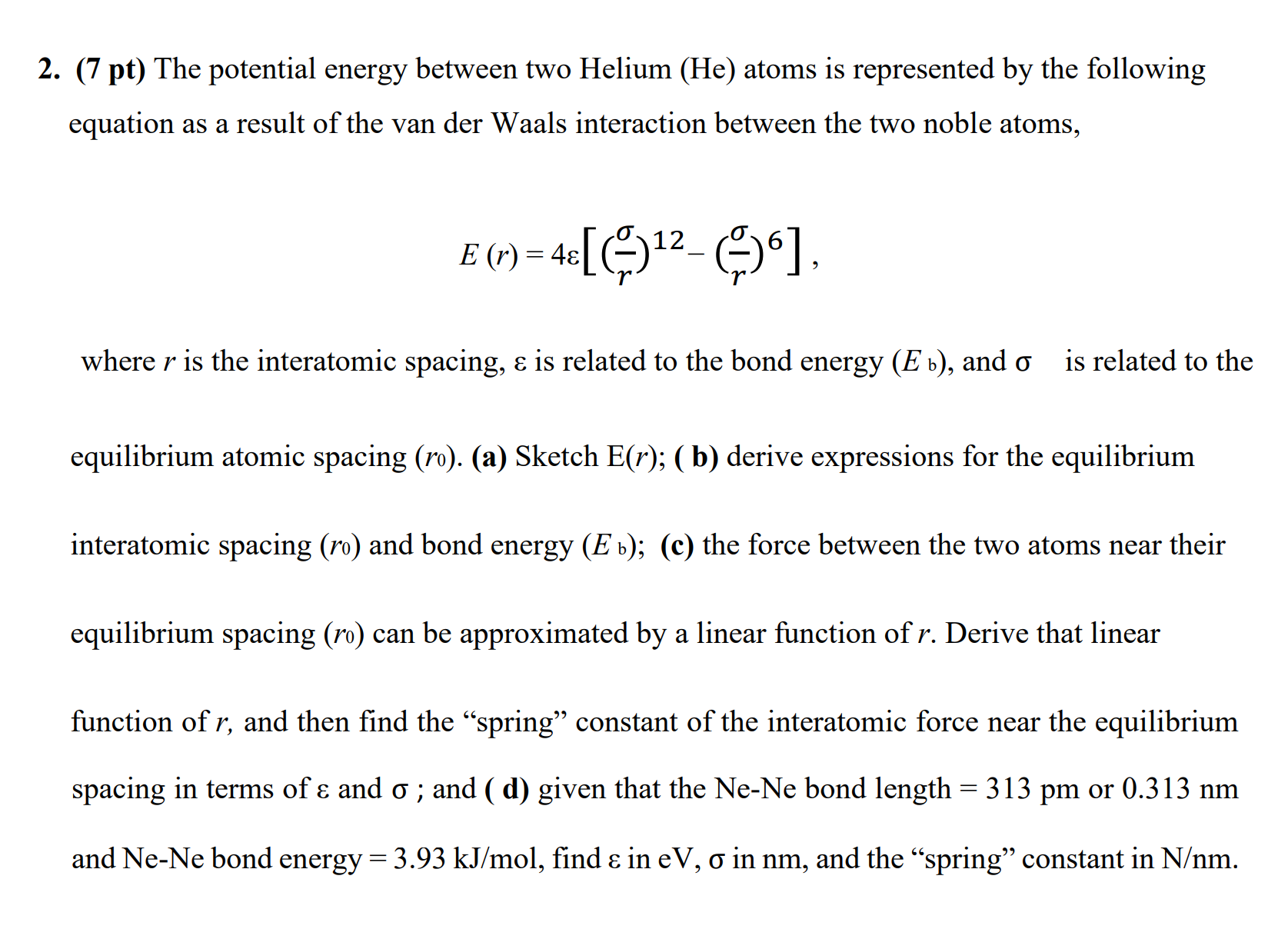
2. (7 pt) The potential energy between two Helium (He) atoms is represented by the following equation as a result of the van der Waals interaction between the two noble atoms, 12 E (r) = 4 [6] where r is the interatomic spacing, & is related to the bond energy (E ), and o is related to the equilibrium atomic spacing (ro). (a) Sketch E(r); ( b) derive expressions for the equilibrium interatomic spacing (ro) and bond energy (E b); (c) the force between the two atoms near their equilibrium spacing (ro) can be approximated by a linear function of r. Derive that linear function of r, and then find the "spring" constant of the interatomic force near the equilibrium spacing in terms of & and o; and (d) given that the Ne-Ne bond length = 313 pm or 0.313 nm and Ne-Ne bond energy = 3.93 kJ/mol, find & in eV, o in nm, and the "spring" constant in N/nm.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solution a Equilibrium Interatomic Spacing r Explanation To find the equilibrium interatomic spacing ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


