Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Below is shown a plot of the logarithm (to the base 10) of the diffusion coefficient versus reciprocal of the absolute temperature for
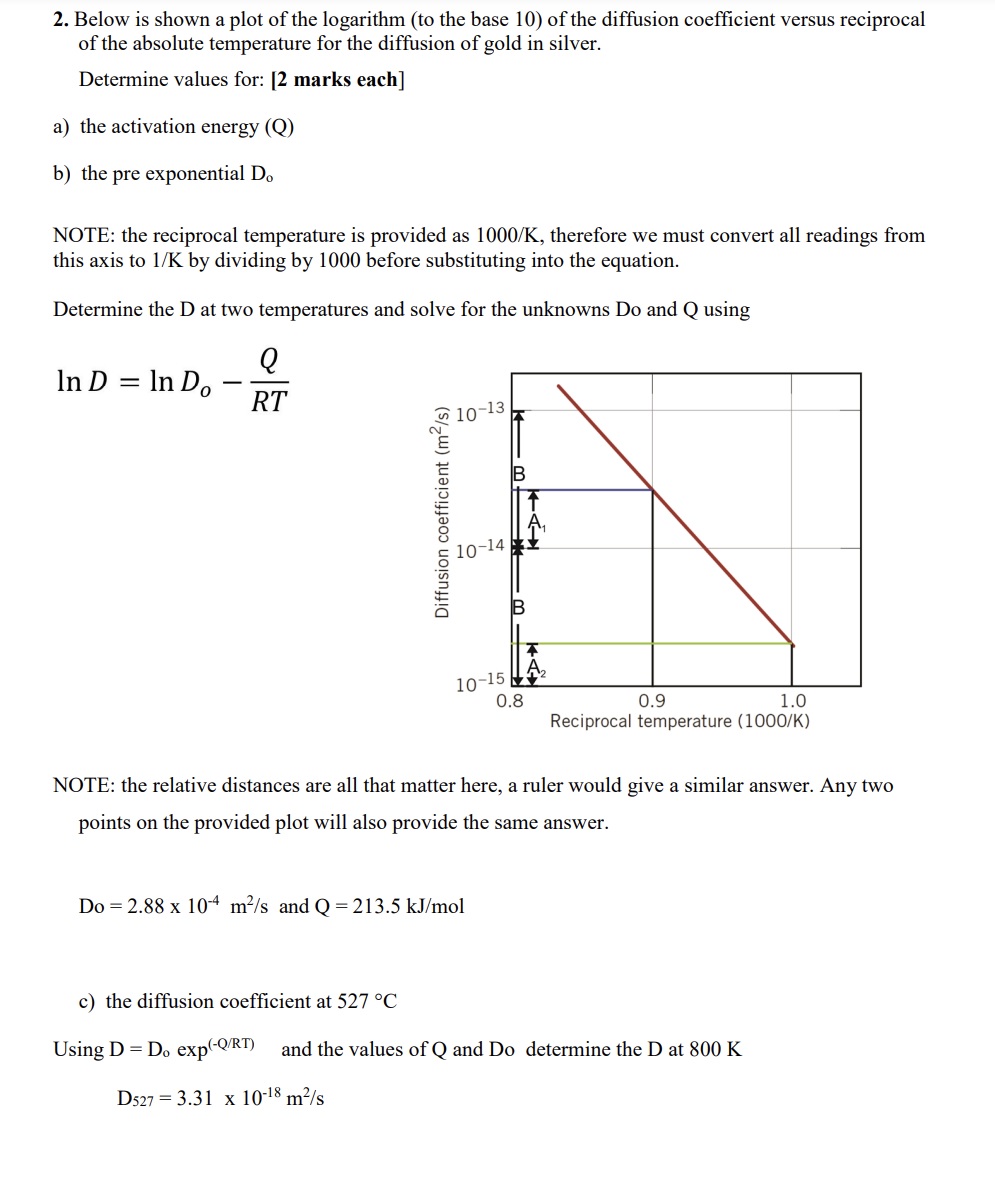
2. Below is shown a plot of the logarithm (to the base 10) of the diffusion coefficient versus reciprocal of the absolute temperature for the diffusion of gold in silver. Determine values for: [2 marks each] a) the activation energy (Q) b) the pre exponential Do NOTE: the reciprocal temperature is provided as 1000/K, therefore we must convert all readings from this axis to 1/K by dividing by 1000 before substituting into the equation. Determine the D at two temperatures and solve for the unknowns Do and Q using In D = In Do - Q RT 1 Diffusion coefficient (m/s) 10-14 m KA 10-15 0.8 0.9 1.0 Reciprocal temperature (1000/K) NOTE: the relative distances are all that matter here, a ruler would give a similar answer. Any two points on the provided plot will also provide the same answer. Do 2.88 x 10 m/s and Q = 213.5 kJ/mol c) the diffusion coefficient at 527 C Using D = Do exp(-Q/RT) and the values of Q and Do determine the D at 800 K D527 3.31 x 10-18 m/s
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


