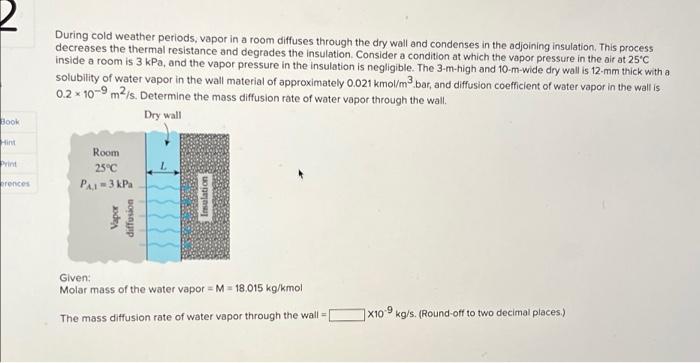
2 During cold weather periods, vapor in a room diffuses through the dry wall and condenses in the adjoining insulation. This process decreases the thermal resistance and degrades the Insulation. Consider a condition at which the vapor pressure in the air at 25C inside a room is 3 kPa, and the vapor pressure in the insulation is negligible. The 3-m-high and 10-m-wide dry wall is 12 mm thick with a solubility of water vapor in the wall material of approximately 0.021 kmol/m2 bar, and diffusion coefficient of water vapor in the wall is 0.2 x 10-9m2/s. Determine the mass diffusion rate of water vapor through the wall Dry wall BOOK Hint Room 25C PX,1-3 kPa rences Insulation Vapor diffusion Given: Molar mass of the water vapor = M = 18.015 kg/kmol The mass diffusion rate of water vapor through the wall X10kg/s. (Round-off to two decimal places) Hydrogen gas at 85C is maintained at constant pressures of 11 atm and 4.2 atm on opposite sides of a 0.1-mm-thick nickel wall. Determine the molar diffusion rate per unit area through the nickel wall. Nickel wall Puls Hydrogen 85 C Hydrogen SC Pw Given: Binary diffusion coefficient for hydrogen in the nickel at 85C is 1.20 x10-12 m2/s. The solubility of H2 in nickel at 85*C is 0.00901 kmol/mYbar. x10-10 kmol/s-m2 (Round-off to two decimal places.) HIP During cold weather periods, vapor in a room diffuses through the dry wall and condenses in the adjoining insulation. This process decreases the thermal resistance and degrades the insulation. Consider a condition at which the vapor pressure in the air at 25C inside a room is 3 kPa, and the vapor pressure in the insulation is negligible. The 3-m-high and 10-m-wide dry wall is 12-mm thick with a solubility of water vapor in the wall material of approximately 0.021 kmol/m2 bar, and diffusion coefficient of water vapor in the wall is 0.2 x 10-9 m2/s. Determine the mass diffusion rate of water vapor through the wall. Dry wall Room 25C PA1 = 3 kPa Vapor diffusion 3 Insulation Given: Molar mass of the water vapor = M = 18.015 kg/kmol The mass diffusion rate of water vapor through the wall X10-9 kg/s. (Round-off to two decimal places)