Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2) For the reaction below, several initial rate experiments are performed and the data are summarized in the table. 2HgCl2 + C2O42 2C1+ 2CO2
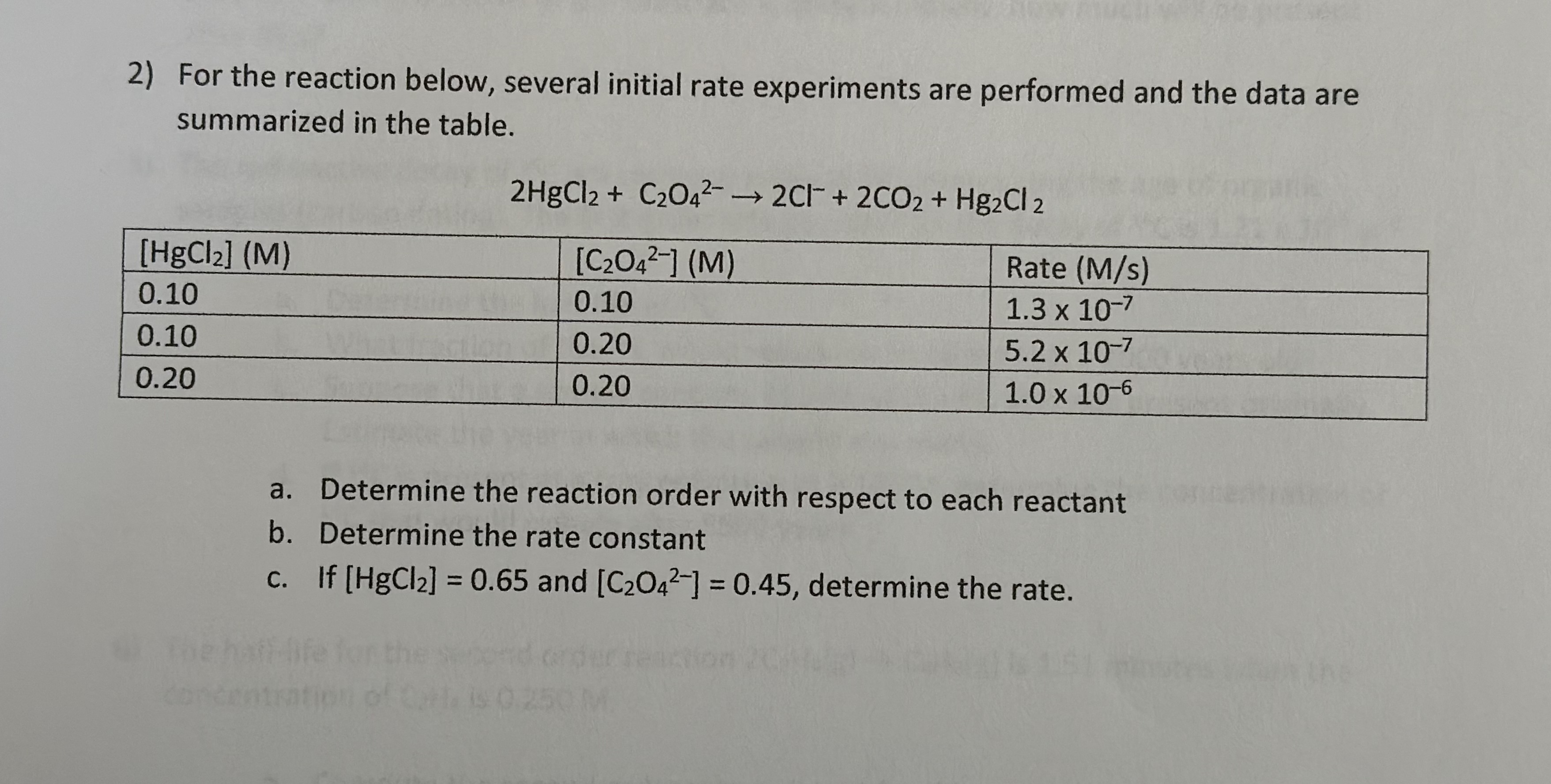
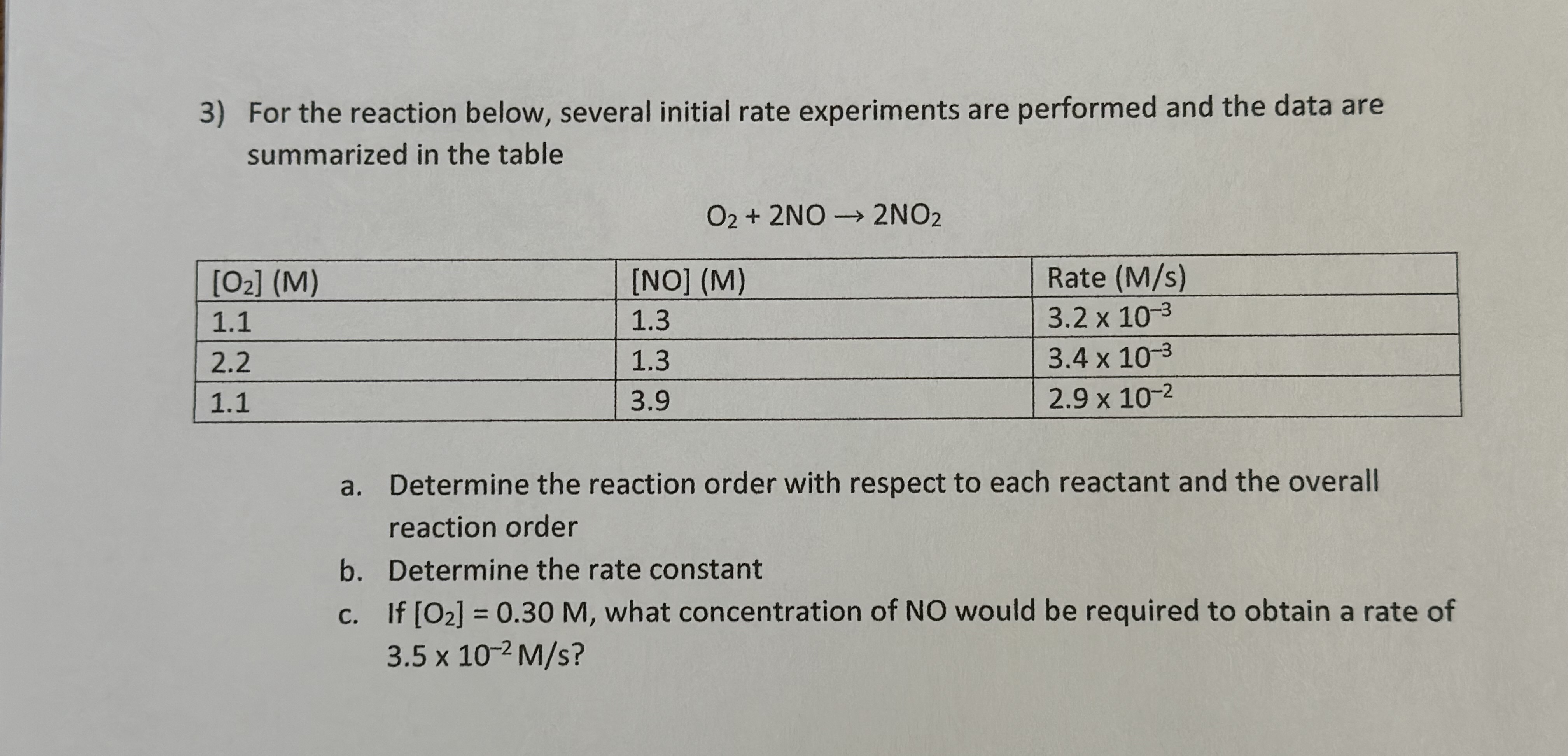
2) For the reaction below, several initial rate experiments are performed and the data are summarized in the table. 2HgCl2 + C2O42 2C1+ 2CO2 + Hg2Cl 2 [HgCl2] (M) 0.10 0.10 0.20 concen [C2042] (M) 0.10 0.20 0.20 Rate (M/s) 1.3 x 10-7 5.2 x 10-7 1.0 x 10-6 a. Determine the reaction order with respect to each reactant b. c. Determine the rate constant If [HgCl] = 0.65 and [C2042] = 0.45, determine the rate. 3) For the reaction below, several initial rate experiments are performed and the data are summarized in the table [02] (M) 1.1 2.2 1.1 O2+2NO 2NO2 [NO] (M) Rate (M/s) 1.3 3.2 x 10-3 1.3 3.9 3.4 x 10-3 2.9 x 10-2 a. Determine the reaction order with respect to each reactant and the overall reaction order b. Determine the rate constant c. If [02] = 0.30 M, what concentration of NO would be required to obtain a rate of 3.5 x 10-2 M/s?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


