Question: 2. In a laboratory experiment you react 20.0 mL of 25% by mass C2H&N2 with 8.000 NiCl2.6H20 to obtain the product Ni(NH2CH2CHNH213Cl2 complex. The
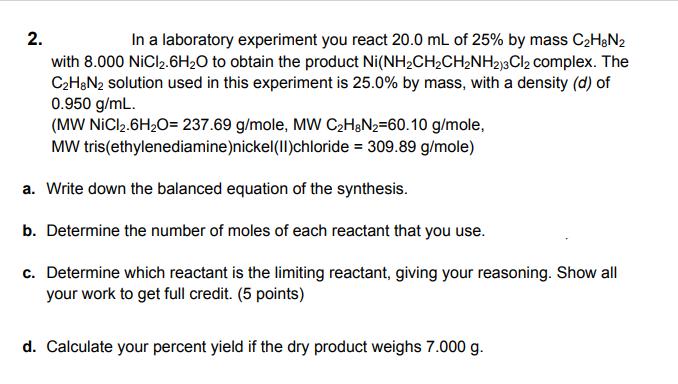
2. In a laboratory experiment you react 20.0 mL of 25% by mass C2H&N2 with 8.000 NiCl2.6H20 to obtain the product Ni(NH2CH2CHNH213Cl2 complex. The C2H&N2 solution used in this experiment is 25.0% by mass, with a density (d) of 0.950 g/mL. (MW NICI2.6H20= 237.69 g/mole, MW C2H&N2=60.10 g/mole, MW tris(ethylenediamine)nickel(II)chloride = 309.89 g/mole) a. Write down the balanced equation of the synthesis. b. Determine the number of moles of each reactant that you use. c. Determine which reactant is the limiting reactant, giving your reasoning. Show all your work to get full credit. (5 points) d. Calculate your percent yield if the dry product weighs 7.000 g.
Step by Step Solution
3.47 Rating (167 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


