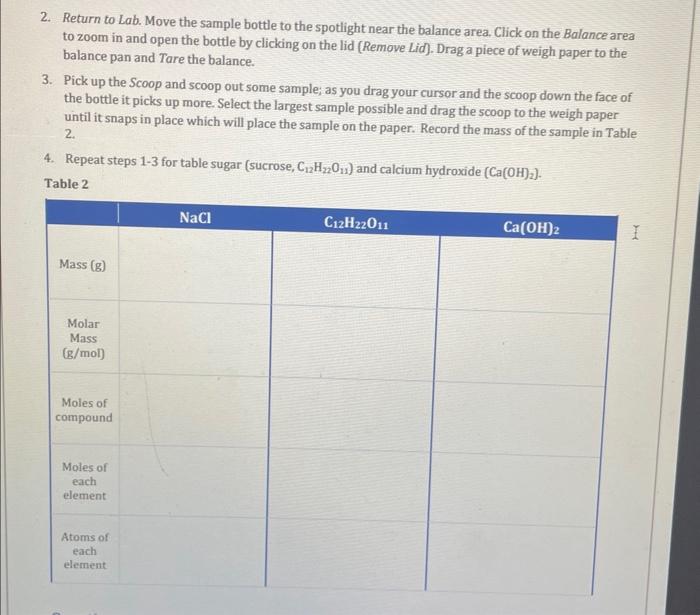
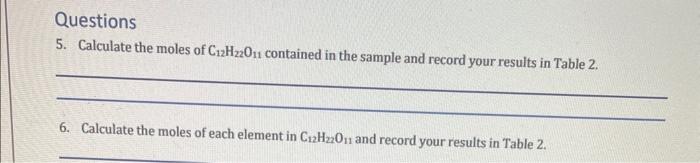
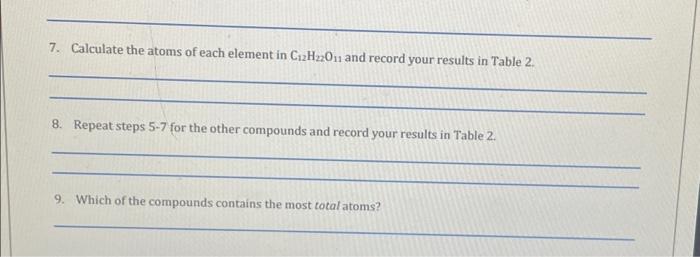
2. Return to Lab. Move the sample bottle to the spotlight near the balance area. Click on the Balance area to zoom in and open the bottle by clicking on the lid (Remove Lid). Drag a piece of weigh paper to the balance pan and Tare the balance. 3. Pick up the Scoop and scoop out some sample; as you drag your cursor and the scoop down the face of the bottle it picks up more. Select the largest sample possible and drag the scoop to the weigh paper until it snaps in place which will place the sample on the paper. Record the mass of the sample in Table 2. 4. Repeat steps 1-3 for table sugar (sucrose, C12H22O11) and calcium hydroxide (Ca(OH)2). Table 2 Questions 5. Calculate the moles of C12H22O11 contained in the sample and record your results in Table 2 . 6. Calculate the moles of each element in C12H22O11 and record your results in Table 2 . 7. Calculate the atoms of each element in C12H22O11 and record your results in Table 2 . 8. Repeat steps 5-7 for the other compounds and record your results in Table 2 . 9. Which of the compounds contains the most total atoms? 2. Return to Lab. Move the sample bottle to the spotlight near the balance area. Click on the Balance area to zoom in and open the bottle by clicking on the lid (Remove Lid). Drag a piece of weigh paper to the balance pan and Tare the balance. 3. Pick up the Scoop and scoop out some sample; as you drag your cursor and the scoop down the face of the bottle it picks up more. Select the largest sample possible and drag the scoop to the weigh paper until it snaps in place which will place the sample on the paper. Record the mass of the sample in Table 2. 4. Repeat steps 1-3 for table sugar (sucrose, C12H22O11) and calcium hydroxide (Ca(OH)2). Table 2 Questions 5. Calculate the moles of C12H22O11 contained in the sample and record your results in Table 2 . 6. Calculate the moles of each element in C12H22O11 and record your results in Table 2 . 7. Calculate the atoms of each element in C12H22O11 and record your results in Table 2 . 8. Repeat steps 5-7 for the other compounds and record your results in Table 2 . 9. Which of the compounds contains the most total atoms