Answered step by step
Verified Expert Solution
Question
1 Approved Answer
also if you know any helpful chem tips please leave them. The reaction between potassium superoxide, KO2, and CO2, 4KO2+2CO22K2CO3+3O2 How many moles of O2
also if you know any helpful chem tips please leave them.
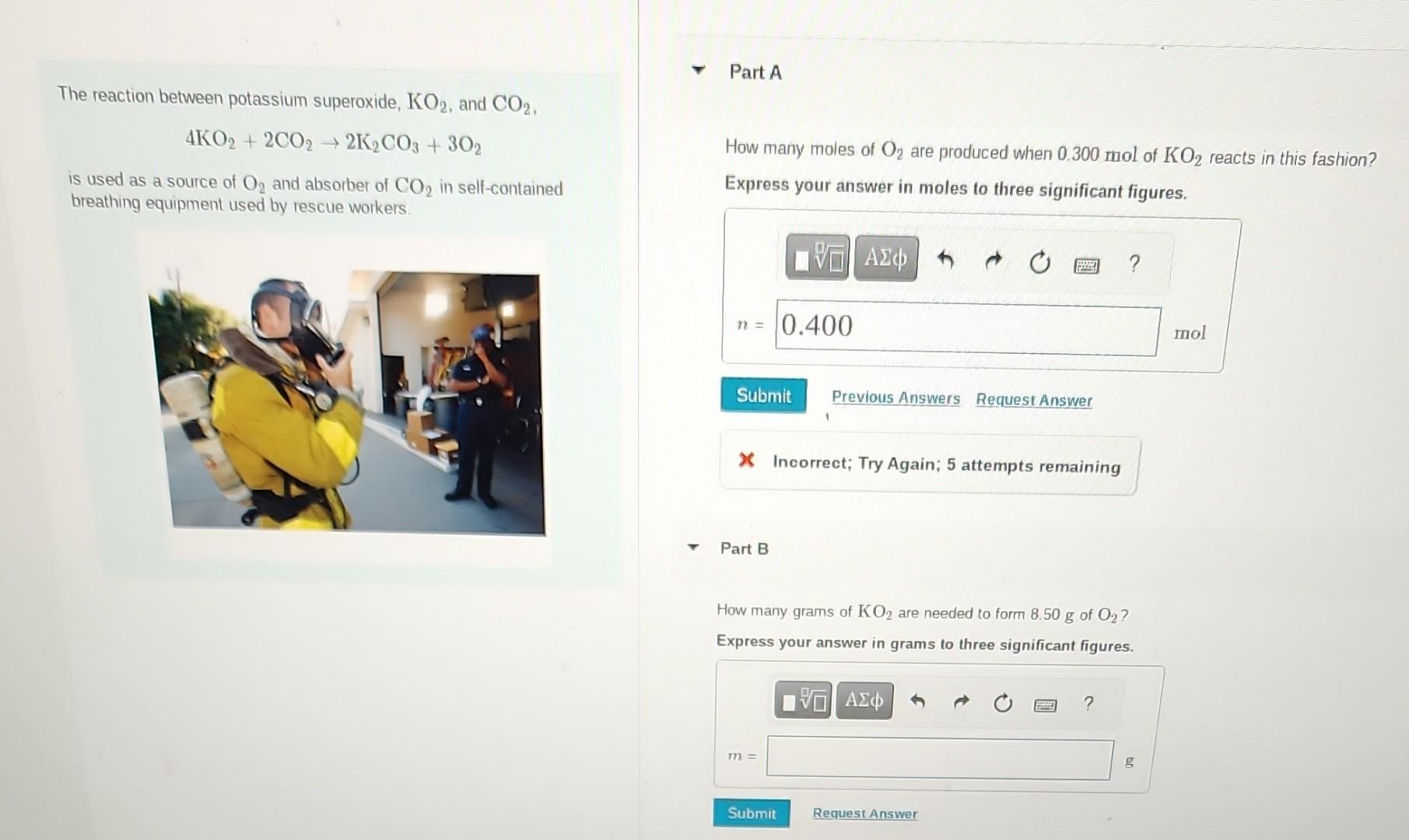
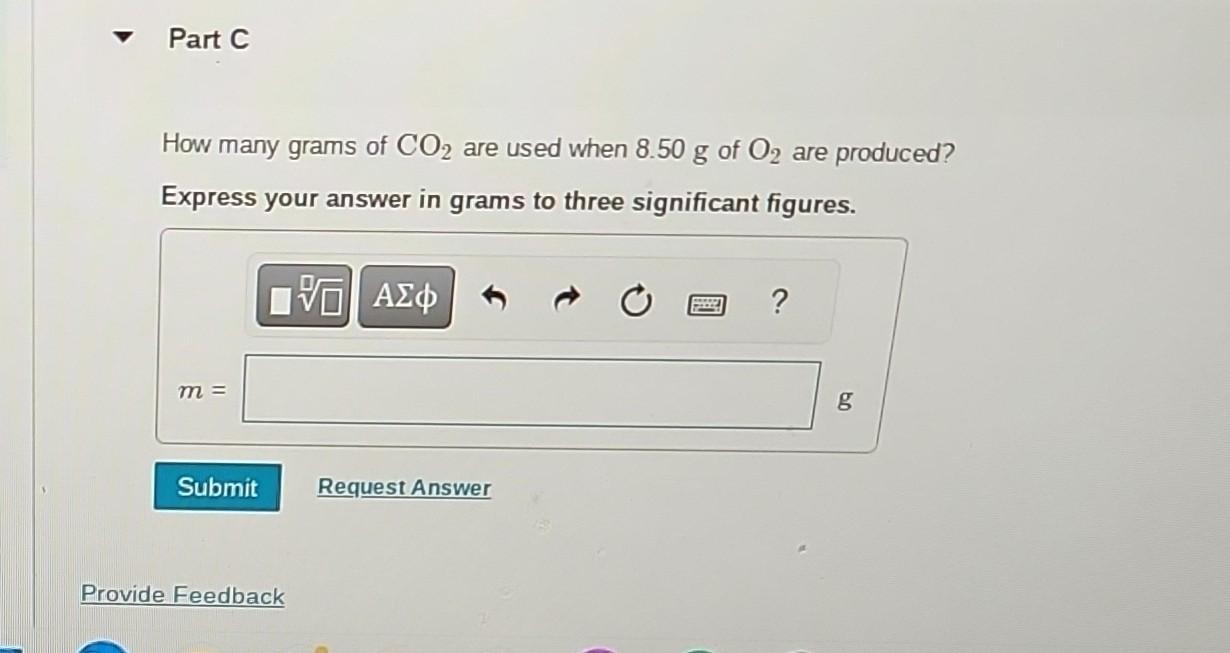
The reaction between potassium superoxide, KO2, and CO2, 4KO2+2CO22K2CO3+3O2 How many moles of O2 are produced when 0.300mol of KO2 reacts in this fashion? is used as a source of O2 and absorber of CO2 in self-contained breathing equipment used by rescue workers. Express your answer in moles to three significant figures. x Incorrect; Try Again; 5 attempts remaining Part B How many grams of KO2 are needed to form 8.50g of O2 ? Express your answer in grams to three significant figures. How many grams of CO2 are used when 8.50g of O2 are produced? Express your answer in grams to three significant figures
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


