Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Synthesis gas may be produced by the catalytic reforming of methane with steam 1: CH4(g)+H2O(g)CO(g)+3H2(g) However the following side reaction can also take place
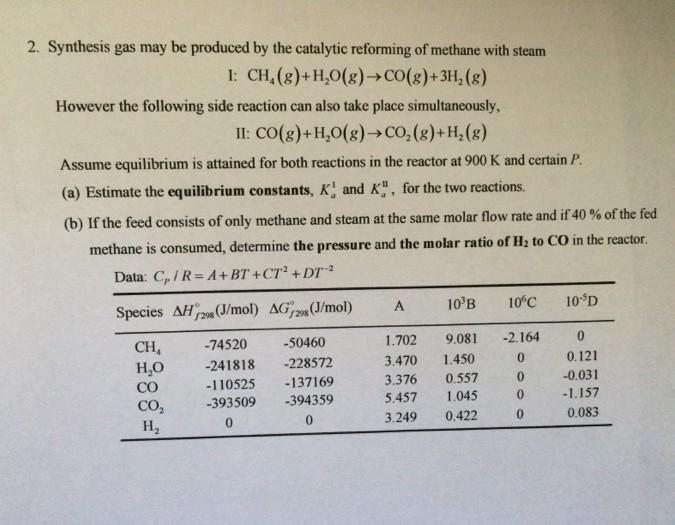
2. Synthesis gas may be produced by the catalytic reforming of methane with steam 1: CH4(g)+H2O(g)CO(g)+3H2(g) However the following side reaction can also take place simultaneously, II:CO(g)+H2O(g)CO2(g)+H2(g) Assume equilibrium is attained for both reactions in the reactor at 900K and certain P. (a) Estimate the equilibrium constants, Ka1 and KaII, for the two reactions. (b) If the feed consists of only methane and steam at the same molar flow rate and if 40% of the fed methane is consumed, determine the pressure and the molar ratio of H2 to CO in the reactor. Data: CD/R=A+BT+CT2+DT2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


