Question
2. The following results were obtained when a sample of gas was tested in a Junker's gas calorimeter: Gas burnt = 0.03 m Gas
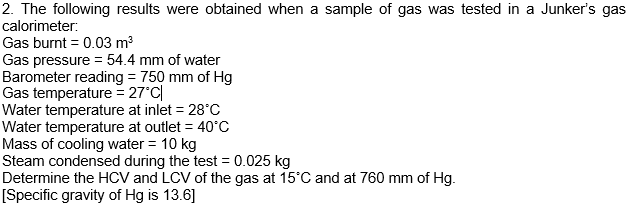
2. The following results were obtained when a sample of gas was tested in a Junker's gas calorimeter: Gas burnt = 0.03 m Gas pressure = 54.4 mm of water Barometer reading = 750 mm of Hg Gas temperature = 27C| Water temperature at inlet = 28C Water temperature at outlet = 40C Mass of cooling water = 10 kg Steam condensed during the test = 0.025 kg Determine the HCV and LCV of the gas at 15C and at 760 mm of Hg. [Specific gravity of Hg is 13.6]
Step by Step Solution
3.39 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
Answer 1 The mass of the sample of gas used in the experiment can be calculated as follows Mass of g...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Peter Atkins
7th Edition
978-0716735397, 716735393, 978-0716743880
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



