Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. The molar mass transfer rate from a 30mm diameter and 5mm length naphthalene cylinder placed in stagnant air at 85.8C and atmospheric pressure is
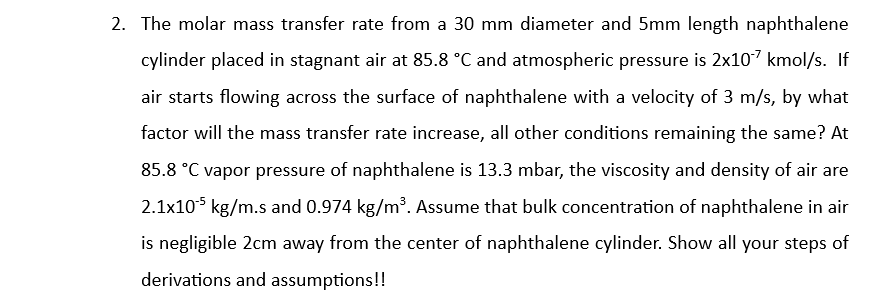 2. The molar mass transfer rate from a 30mm diameter and 5mm length naphthalene cylinder placed in stagnant air at 85.8C and atmospheric pressure is 2107kmol/s. If air starts flowing across the surface of naphthalene with a velocity of 3m/s, by what factor will the mass transfer rate increase, all other conditions remaining the same? At 85.8C vapor pressure of naphthalene is 13.3 mbar, the viscosity and density of air are 2.1105kg/m.s and 0.974kg/m3. Assume that bulk concentration of naphthalene in air is negligible 2cm away from the center of naphthalene cylinder. Show all your steps of derivations and assumptions
2. The molar mass transfer rate from a 30mm diameter and 5mm length naphthalene cylinder placed in stagnant air at 85.8C and atmospheric pressure is 2107kmol/s. If air starts flowing across the surface of naphthalene with a velocity of 3m/s, by what factor will the mass transfer rate increase, all other conditions remaining the same? At 85.8C vapor pressure of naphthalene is 13.3 mbar, the viscosity and density of air are 2.1105kg/m.s and 0.974kg/m3. Assume that bulk concentration of naphthalene in air is negligible 2cm away from the center of naphthalene cylinder. Show all your steps of derivations and assumptions Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


