Answered step by step
Verified Expert Solution
Question
1 Approved Answer
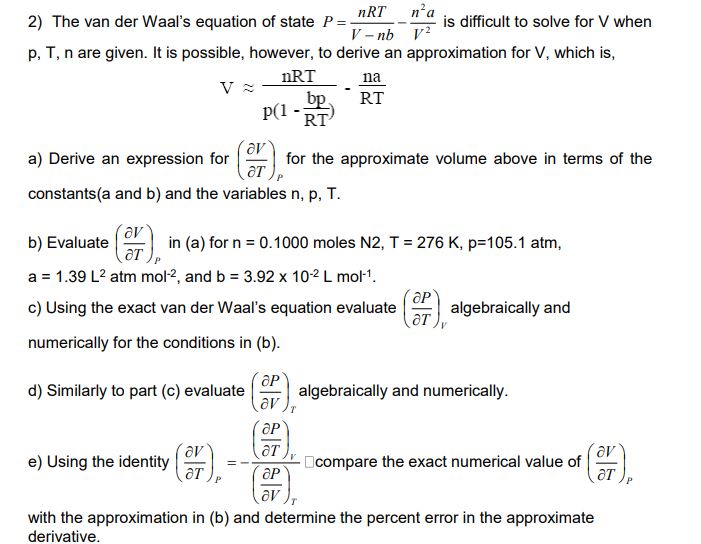
2 ) The van der Waal s equation of state 2 2 V n a V nb nRT P is difficult to solve for V
The van der Waals equation of state
V
n a
V nb
nRT P
is difficult to solve for V when
p T n are given. It is possible, however, to derive an approximation for V which is
a Derive an expression for
T P
V
for the approximate volume above in terms of the
constantsa and b and the variables n p T
b Evaluate
T P
V
in a for n moles N T K p atm,
a L atm mol
and b x L mol
c Using the exact van der Waals equation evaluate
T V
P
algebraically and
numerically for the conditions in b
d Similarly to part c evaluate
V T
P
algebraically and numerically.
e Using the identity
T
V
P
V
P
T
P
T
V
compare the exact numerical value of
T P
V
with the approximation in b and determine the percent error in the approximate
derivative.The van der Waal's equation of state is difficult to solve for when
are given. It is possible, however, to derive an approximation for which is
~~
a Derive an expression for for the approximate volume above in terms of the
constants and and the variables
b Evaluate in a for moles atm,
and
c Using the exact van der Waal's equation evaluate algebraically and
numerically for the conditions in b
d Similarly to part c evaluate algebraically and numerically.
e Using the identity compare the exact numerical value of
with the approximation in b and determine the percent error in the approximate
derivative.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


