Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Two kilograms of water at 125C is in a piston-cylinder assembly. The water undergoes two processes in series: (1) constant-volume heating followed by
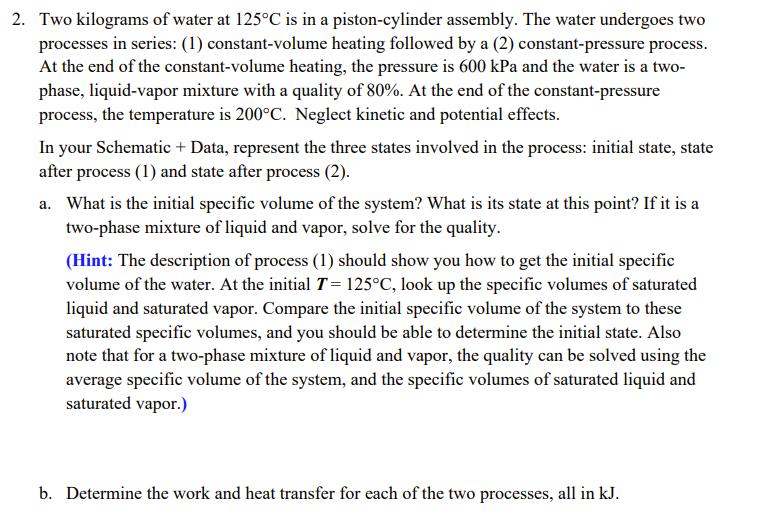
2. Two kilograms of water at 125C is in a piston-cylinder assembly. The water undergoes two processes in series: (1) constant-volume heating followed by a (2) constant-pressure process. At the end of the constant-volume heating, the pressure is 600 kPa and the water is a two- phase, liquid-vapor mixture with a quality of 80%. At the end of the constant-pressure process, the temperature is 200C. Neglect kinetic and potential effects. In your Schematic + Data, represent the three states involved in the process: initial state, state after process (1) and state after process (2). a. What is the initial specific volume of the system? What is its state at this point? If it is a two-phase mixture of liquid and vapor, solve for the quality. (Hint: The description of process (1) should show you how to get the initial specific volume of the water. At the initial T = 125C, look up the specific volumes of saturated liquid and saturated vapor. Compare the initial specific volume of the system to these saturated specific volumes, and you should be able to determine the initial state. Also note that for a two-phase mixture of liquid and vapor, the quality can be solved using the average specific volume of the system, and the specific volumes of saturated liquid and saturated vapor.) b. Determine the work and heat transfer for each of the two processes, all in kJ.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the initial specific volume and state of the system we can use the fact that the water is initially at a temperature of 125C and undergoes con...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


