Answered step by step
Verified Expert Solution
Question
1 Approved Answer
23. 24. 25. 26. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. D Temperature A) Bo(s) has a
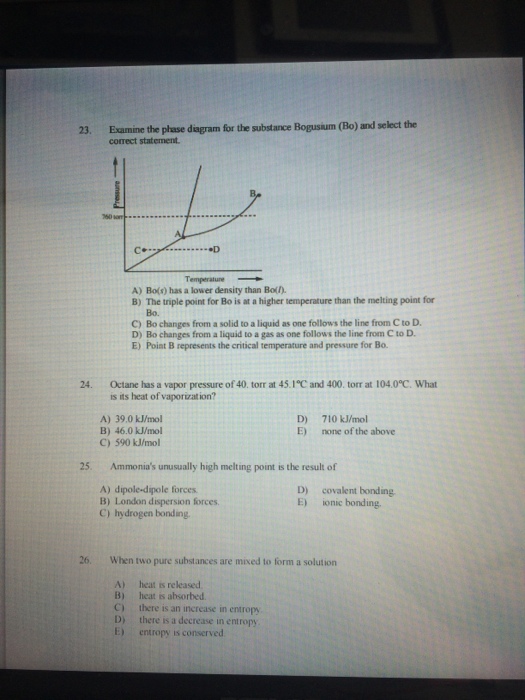
23. 24. 25. 26. Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. D Temperature A) Bo(s) has a lower density than Bo(). B) The triple point for Bo is at a higher temperature than the melting point for Bo. C) Bo changes from a solid to a liquid as one follows the line from C to D. D) Bo changes from a liquid to a gas as one follows the line from C to D. E) Point B represents the critical temperature and pressure for Bo. Octane has a vapor pressure of 40. torr at 45.1C and 400. torr at 104.0C. What is its heat of vaporization? A) 39.0 kJ/mol B) 46.0 kJ/mol C) 590 kJ/mol D) E) 710 kJ/mol none of the above Ammonia's unusually high melting point is the result of A) dipole-dipole forces. B) London dispersion forces. C) hydrogen bonding. D) E) covalent bonding ionic bonding. When two pure substances are mixed to form a solution A) heat is released. B) heat is absorbed. C) there is an increase in entropy D) there is a decrease in entropy. E) entropy is conserved.
Step by Step Solution
★★★★★
3.39 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


