Question
2Na (s) +2H 2 O (l) 2NaOH (aq) +H 2(g) In this chemical equation, H 2 O is the oxidizingagent. I am confused because on
2Na(s) +2H2O(l)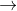 2NaOH(aq) +H2(g)
2NaOH(aq) +H2(g)
In this chemical equation, H2O is the oxidizingagent. I am confused because on the reactant side H2Ohas an oxidation number of 0. How can we tell that it has reducedif there is no H2O to compare to on the product side? Iunderstand that a whole compound can be a reducing or oxidizingagent but how can we say that it is the oxidizing agent if we breakit apart on the product side?
Step by Step Solution
3.44 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
Due to the reaction 40 has been converted to b Conside...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Economics
Authors: R. Glenn Hubbard
6th edition
978-0134797731, 134797736, 978-0134106243
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



