Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3 . 1 0 . An isothermal, irreversible reaction A , - ? k , B takes place in the liquid phase in a constant
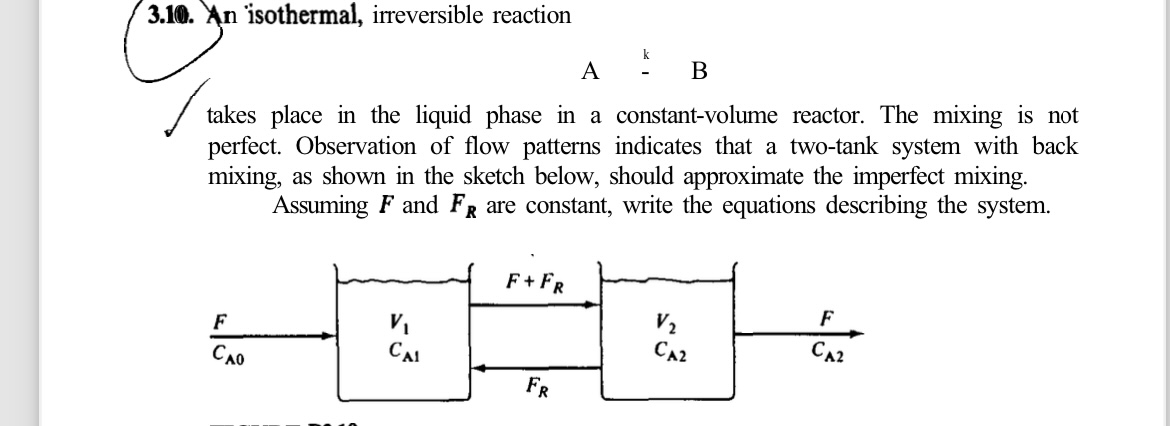
An isothermal, irreversible reaction
takes place in the liquid phase in a constantvolume reactor. The mixing is not perfect. Observation of flow patterns indicates that a twotank system with back mixing, as shown in the sketch below, should approximate the imperfect mixing.
Assuming and are constant, write the equations describing the system.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


