Answered step by step
Verified Expert Solution
Question
1 Approved Answer
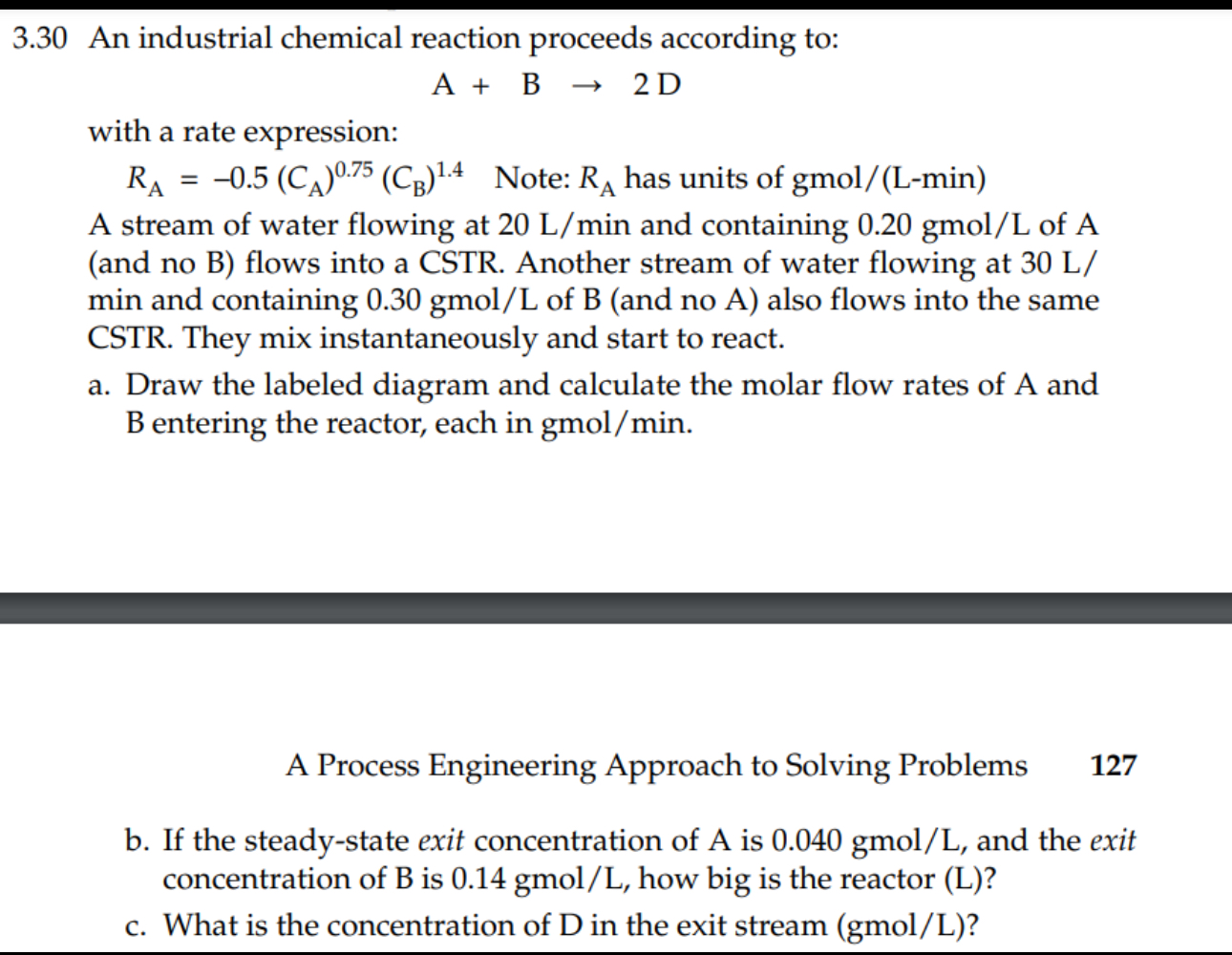
3 . 3 0 An industrial chemical reaction proceeds according to: A + B 2 D with a rate expression: R A = - 0
An industrial chemical reaction proceeds according to:
with a rate expression:
Note: has units gmo
A stream of water flowing at and containing gmo of and no B flows into a CSTR Another stream of water flowing at min and containing gmo of and no also flows into the same CSTR They mix instantaneously and start to react.
a Draw the labeled diagram and calculate the molar flow rates of A and entering the reactor, each in gmo
A Process Engineering Approach to Solving Problems
b If the steadystate exit concentration of A is gmo and the exit concentration of is gmo how big is the reactor
c What is the concentration of in the exit stream
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


