Answered step by step
Verified Expert Solution
Question
1 Approved Answer
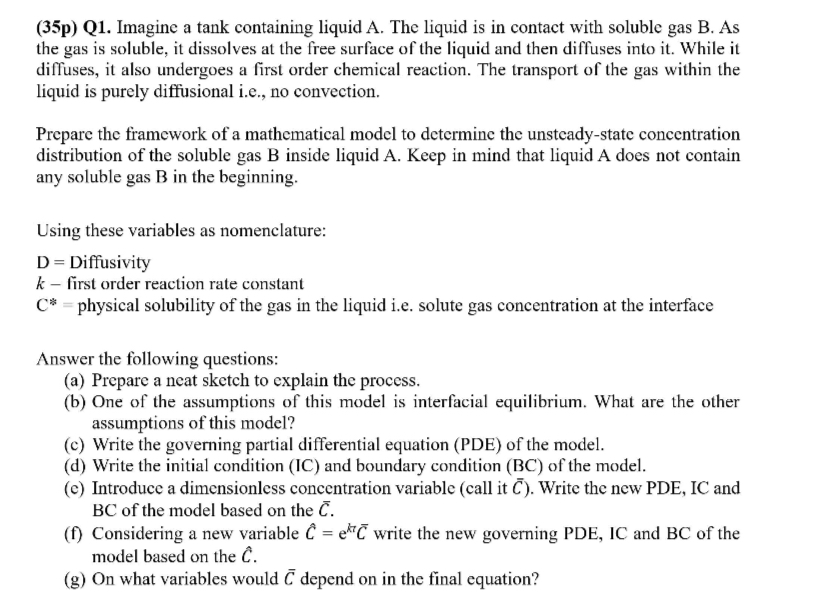
( 3 5 p ) Q 1 . Imagine a tank containing liquid A . The liquid is in contact with soluble gas B .
p Q Imagine a tank containing liquid A The liquid is in contact with soluble gas B As the gas is soluble, it dissolves at the free surface of the liquid and then diffuses into it While it diffuses, it also undergoes a first order chemical reaction. The transport of the gas within the liquid is purely diffusional ie no convection.
Prepare the framework of a mathematical model to determine the unsteadystate concentration distribution of the soluble gas inside liquid Keep in mind that liquid A does not contain any soluble gas in the beginning.
Using these variables as nomenclature:
Diffusivity
first order reaction rate constant
physical solubility of the gas in the liquid ie solute gas concentration at the interface
Answer the following questions:
a Prepare a neat sketch to explain the process.
b One of the assumptions of this model is interfacial equilibrium. What are the other assumptions of this model?
c Write the governing partial differential equation PDE of the model.
d Write the initial condition IC and boundary condition BC of the model.
e Introduce a dimensionless concentration variable call it Write the new PDE, IC and BC of the model based on the
f Considering a new variable hat write the new governing PDE, IC and BC of the model based on the hat
g On what variables would depend on in the final equation?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


