Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. [From old exam] A petrochemical plant has a reactor waste stream containing hydrogen (20 mole%) in methane. The engineers wish to recover the
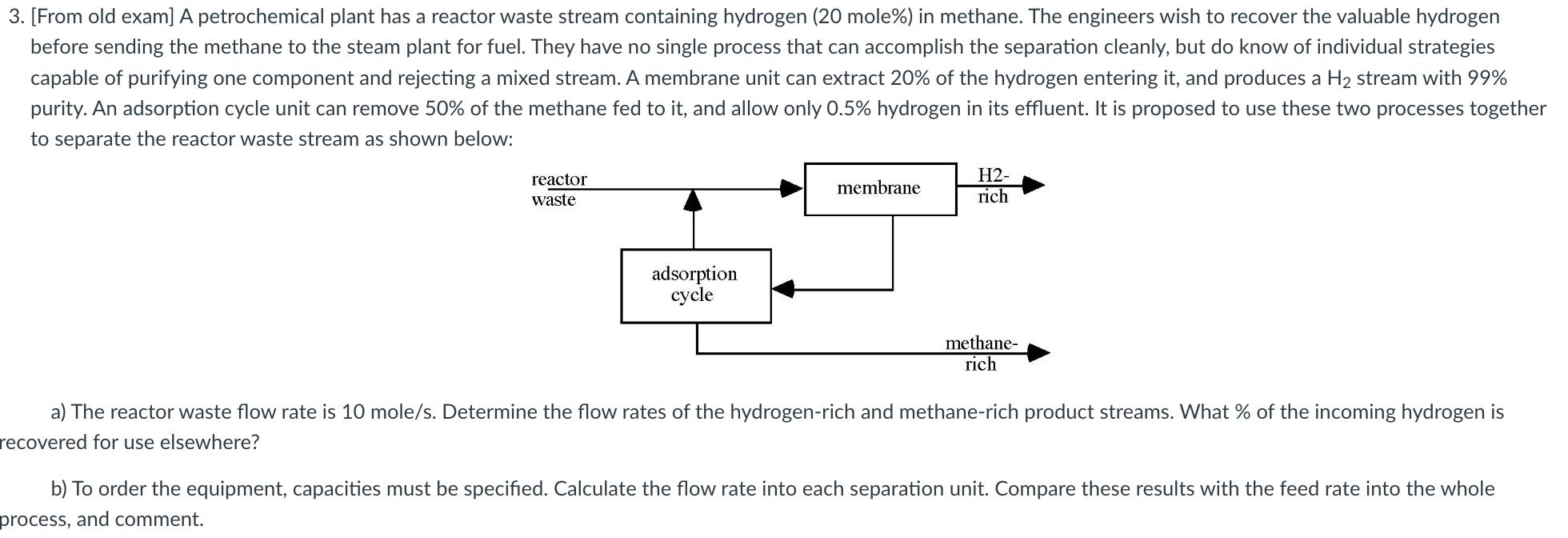
3. [From old exam] A petrochemical plant has a reactor waste stream containing hydrogen (20 mole%) in methane. The engineers wish to recover the valuable hydrogen before sending the methane to the steam plant for fuel. They have no single process that can accomplish the separation cleanly, but do know of individual strategies capable of purifying one component and rejecting a mixed stream. A membrane unit can extract 20% of the hydrogen entering it, and produces a H2 stream with 99% purity. An adsorption cycle unit can remove 50% of the methane fed to it, and allow only 0.5% hydrogen in its effluent. It is proposed to use these two processes together to separate the reactor waste stream as shown below: reactor waste adsorption cycle membrane H2- rich methane- rich a) The reactor waste flow rate is 10 mole/s. Determine the flow rates of the hydrogen-rich and methane-rich product streams. What % of the incoming hydrogen is recovered for use elsewhere? b) To order the equipment, capacities must be specified. Calculate the flow rate into each separation unit. Compare these results with the feed rate into the whole process, and comment.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


