Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. The catalytic isomerization of n-pentane is a reversible reaction given below: n-CsH12 i-CsH12 is carried out in a plug flow reactor using a
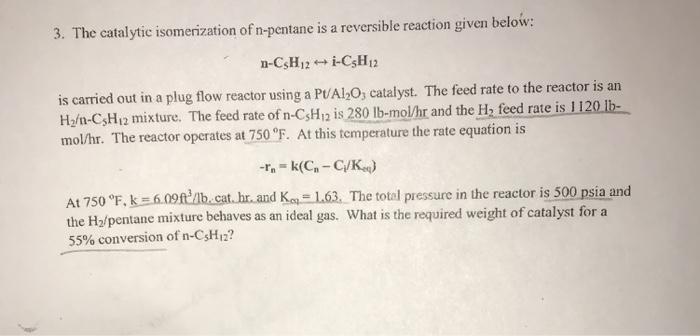
3. The catalytic isomerization of n-pentane is a reversible reaction given below: n-CsH12 i-CsH12 is carried out in a plug flow reactor using a PU/Al2O3 catalyst. The feed rate to the reactor is an H/n-CsH12 mixture. The feed rate of n-CsH12 is 280 lb-mol/hr and the H feed rate is 1120 lb- mol/hr. The reactor operates at 750 F. At this temperature the rate equation is -r-k(C-C/Kq) At 750 F, k=6.09ft/lb. cat. hr. and K-1.63. The total pressure in the reactor is 500 psia and the H/pentane mixture behaves as an ideal gas. What is the required weight of catalyst for a 55% conversion of n-CsH12?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
A great problem in chemical reaction engineering Lets break it down step by step 1 Convert the feed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


