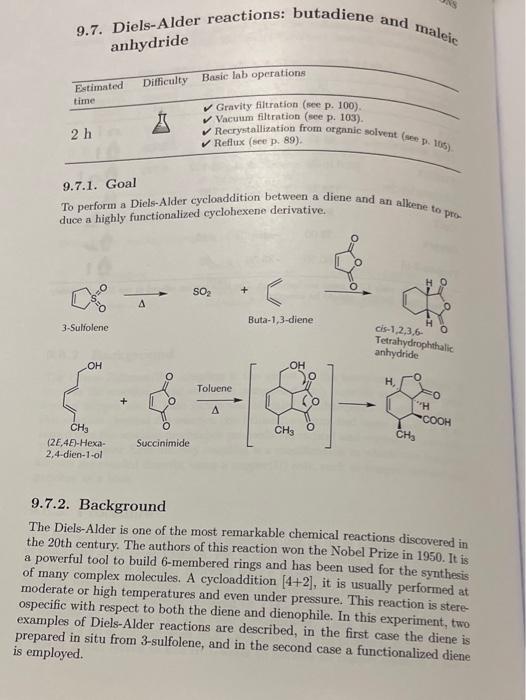
3. The reaction is concerted and stereospecific. Using arrows to show the movement of electrons, draw the mechanism for the formation of the products from part A and part B 9.7. Diels-Alder reactions: butadiene and malejc anhydride 9.7.1. Goal To perform a Diels-Alder cycloaddition between a diene and an alkene to pra duce a highly functionalized cyclohexene derivative. Buta-1,3-diene (2E,4E-Hexa- Succinimide Tetrahydrophthalic antydride 2,4-dien-1-ol 9.7.2. Background The Diels-Alder is one of the most remarkable chemical reactions discovered in the 20th century. The authors of this reaction won the Nobel Prize in 1950. It is a powerful tool to build 6 -membered rings and has been used for the synthesis of many complex molecules. A cycloaddition [4+2], it is usually performed at moderate or high temperatures and even under pressure. This reaction is stere ospecific with respect to both the diene and dienophile. In this experiment, two examples of Diels-Alder reactions are described, in the first case the diene is prepared in situ from 3 -sulfolene, and in the second case a functionalized diene is employed. A) Cycloaddition [4+2] between the maleic anhydride and buta-1,3-diene: In a 100ml round-bottom flask, place 10g of 3-8ulfolene (buta-1,3-diene keep the mixture refluxed for 45min with. Next, add 10ml of xylene and attached at the end of the condenser with a solution stirring. A gas trap is because the reaction releases SO2. Cool the solution containing 5%NaOH, of toluene and activated carbon to discolor the solution r.t. and add 50ml To the filtrate, add 2530ml of hexane, and heat to solution to r.t. and then place it in an ice bath. to 80C. First cool the formed (see refs. [1,2] ). B) Cycloaddition [4+2] between maleic anhydride and E,E-2,4-hexadien-1-ol: Reflux a mixture of 0.40g of maleic anhydride and 0.40g of E,E-2,4-hexadien1-ol in 5ml of toluene for 5min. Let the solution cool slowly to r.t.; a solid will start to appear product, chill in an is the fle precipidry. It can be recrystallized from tolh for 10min. Vacuum filter and air dry. It can be recrystallized from toluene (m.p.=156159C, lit. 161C; see refs. [1,2]). 3. The reaction is concerted and stereospecific. Using arrows to show the movement of electrons, draw the mechanism for the formation of the products from part A and part B 9.7. Diels-Alder reactions: butadiene and malejc anhydride 9.7.1. Goal To perform a Diels-Alder cycloaddition between a diene and an alkene to pra duce a highly functionalized cyclohexene derivative. Buta-1,3-diene (2E,4E-Hexa- Succinimide Tetrahydrophthalic antydride 2,4-dien-1-ol 9.7.2. Background The Diels-Alder is one of the most remarkable chemical reactions discovered in the 20th century. The authors of this reaction won the Nobel Prize in 1950. It is a powerful tool to build 6 -membered rings and has been used for the synthesis of many complex molecules. A cycloaddition [4+2], it is usually performed at moderate or high temperatures and even under pressure. This reaction is stere ospecific with respect to both the diene and dienophile. In this experiment, two examples of Diels-Alder reactions are described, in the first case the diene is prepared in situ from 3 -sulfolene, and in the second case a functionalized diene is employed. A) Cycloaddition [4+2] between the maleic anhydride and buta-1,3-diene: In a 100ml round-bottom flask, place 10g of 3-8ulfolene (buta-1,3-diene keep the mixture refluxed for 45min with. Next, add 10ml of xylene and attached at the end of the condenser with a solution stirring. A gas trap is because the reaction releases SO2. Cool the solution containing 5%NaOH, of toluene and activated carbon to discolor the solution r.t. and add 50ml To the filtrate, add 2530ml of hexane, and heat to solution to r.t. and then place it in an ice bath. to 80C. First cool the formed (see refs. [1,2] ). B) Cycloaddition [4+2] between maleic anhydride and E,E-2,4-hexadien-1-ol: Reflux a mixture of 0.40g of maleic anhydride and 0.40g of E,E-2,4-hexadien1-ol in 5ml of toluene for 5min. Let the solution cool slowly to r.t.; a solid will start to appear product, chill in an is the fle precipidry. It can be recrystallized from tolh for 10min. Vacuum filter and air dry. It can be recrystallized from toluene (m.p.=156159C, lit. 161C; see refs. [1,2])