Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. You want to test the rate expression that you derived in problem 1, where C P + B, against reactor testing data using
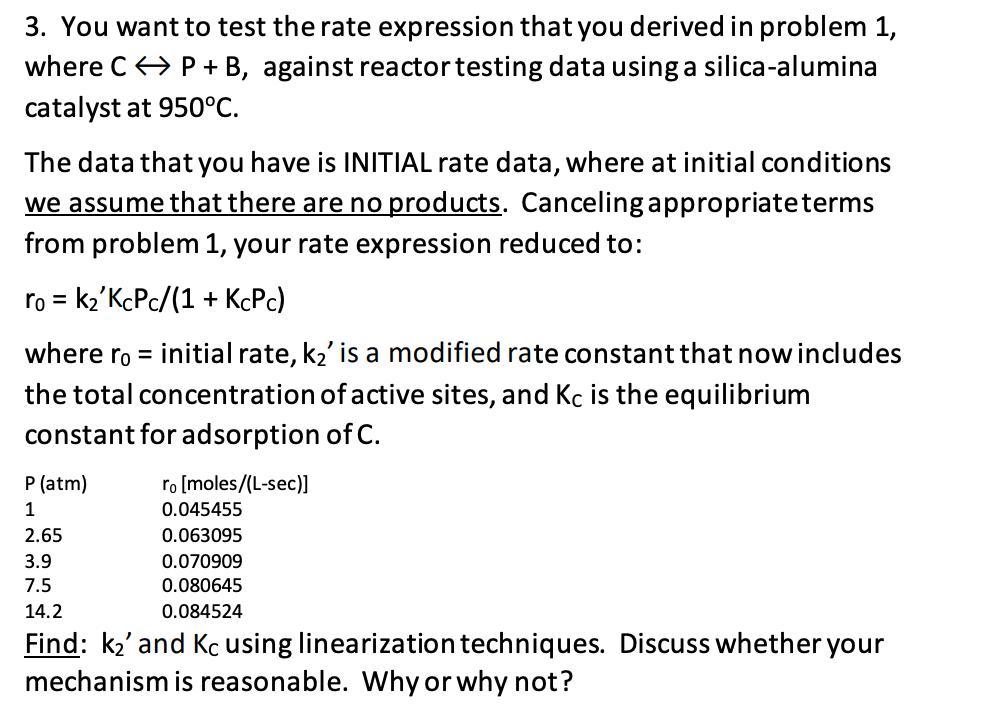
3. You want to test the rate expression that you derived in problem 1, where C P + B, against reactor testing data using a silica-alumina catalyst at 950C. The data that you have is INITIAL rate data, where at initial conditions we assume that there are no products. Canceling appropriate terms from problem 1, your rate expression reduced to: ro = k'KcPc/(1+ KcPc) where ro = initial rate, k' is a modified rate constant that now includes the total concentration of active sites, and Kc is the equilibrium constant for adsorption of C. ro [moles/(L-sec)] 0.045455 0.063095 0.070909 0.080645 0.084524 Find: k' and Kc using linearization techniques. Discuss whether your mechanism is reasonable. Why or why not? P (atm) 1 2.65 3.9 7.5 14.2
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Regarding the discussion on whether the rate expression fits the data well we can analyze the follow...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


