Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3.25. A mixture is 10.0 mole% methyl alcohol, 75.0 mole% methyl acetate (C2H4O2), and 15.0 mole% acetic acid. Calculate the mass fractions of each compound.
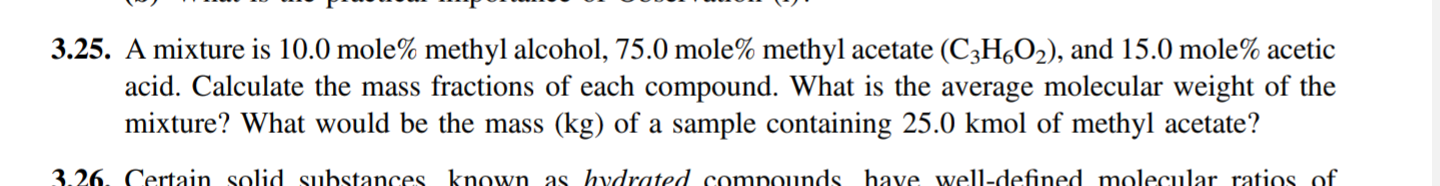
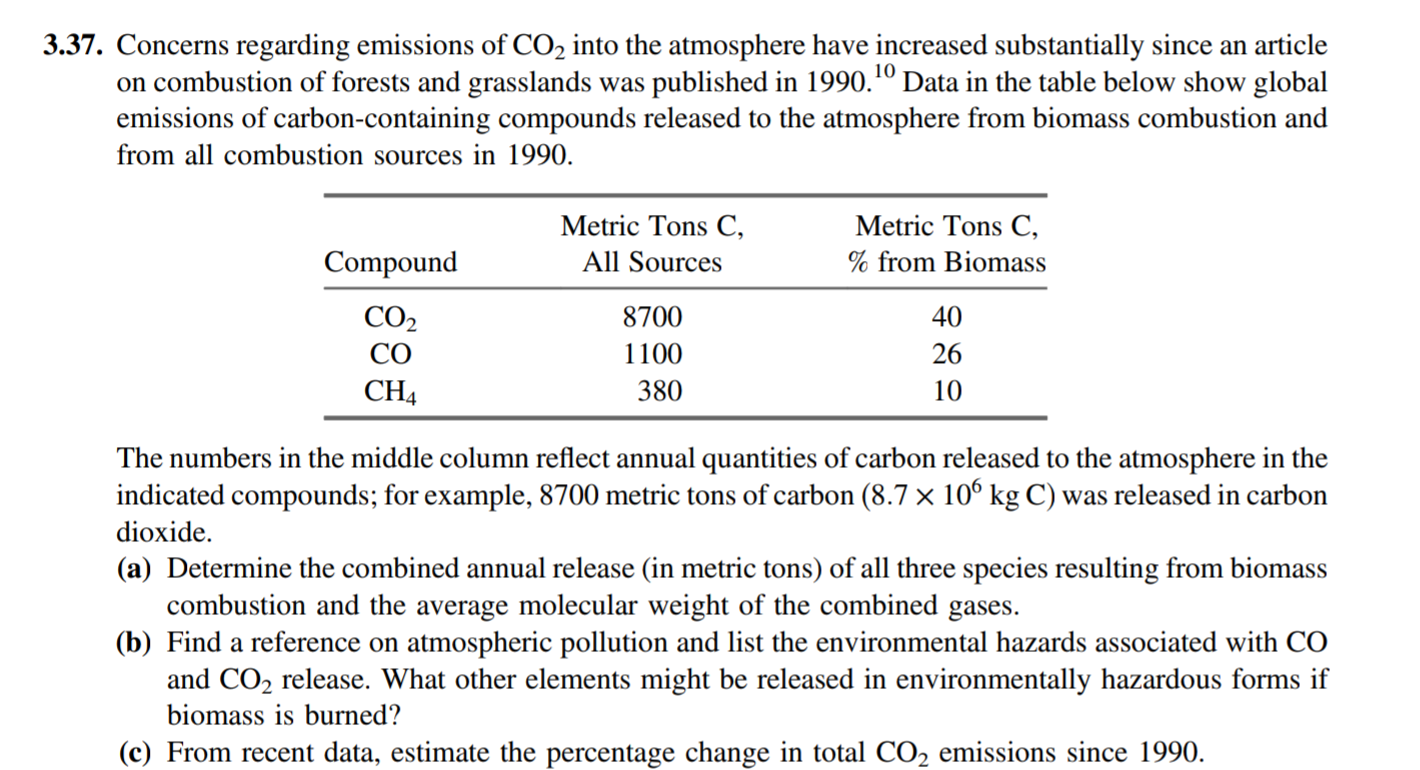
3.25. A mixture is 10.0 mole% methyl alcohol, 75.0 mole% methyl acetate (C2H4O2), and 15.0 mole% acetic acid. Calculate the mass fractions of each compound. What is the average molecular weight of the mixture? What would be the mass (kg) of a sample containing 25.0 kmol of methyl acetate? 3.26. Certain solid substances known as hydrated compounds have well-defined molecular ratios of 3.37. Concerns regarding emissions of CO2 into the atmosphere have increased substantially since an article on combustion of forests and grasslands was published in 1990.10 Data in the table below show global emissions of carbon-containing compounds released to the atmosphere from biomass combustion and from all combustion sources in 1990. Metric Tons C, All Sources Metric Tons C, % from Biomass Compound CO2 CO CH4 8700 1100 380 40 26 10 The numbers in the middle column reflect annual quantities of carbon released to the atmosphere in the indicated compounds; for example, 8700 metric tons of carbon (8.7 x 106 kg C) was released in carbon dioxide. (a) Determine the combined annual release (in metric tons) of all three species resulting from biomass combustion and the average molecular weight of the combined gases. (b) Find a reference on atmospheric pollution and list the environmental hazards associated with CO and CO2 release. What other elements might be released in environmentally hazardous forms if biomass is burned? (c) From recent data, estimate the percentage change in total CO2 emissions since 1990
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


