Question
3.3 Calculate the Q, DS, and DSiso of the following process of 1 kg H2O: H2O(1, -10C,100kPa)H2O(s, -10C,100kPa). Given: At 100 kPa, the melting
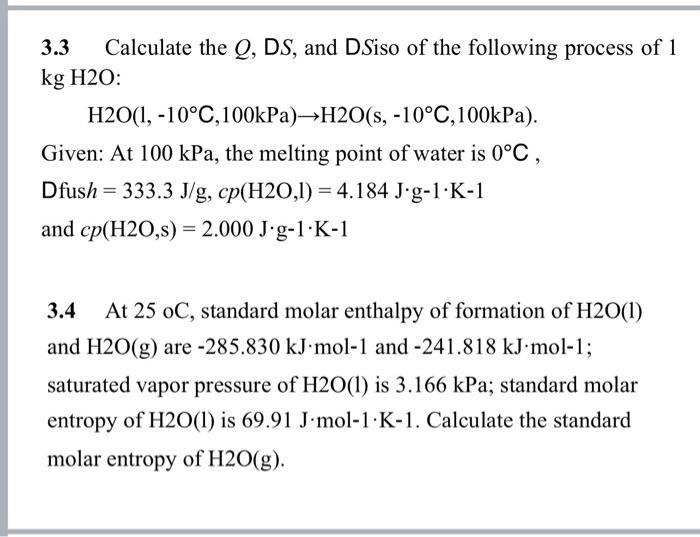
3.3 Calculate the Q, DS, and DSiso of the following process of 1 kg H2O: H2O(1, -10C,100kPa)H2O(s, -10C,100kPa). Given: At 100 kPa, the melting point of water is 0C, Dfush = 333.3 J/g, cp(H2O,1)= 4.184 Jg-1K-1 and cp(H2O,s) = 2.000 J-g-1K-1 3.4 At 25 oC, standard molar enthalpy of formation of H2O(1) and H2O(g) are -285.830 kJ mol-1 and -241.818 kJ.mol-1; saturated vapor pressure of H2O(1) is 3.166 kPa; standard molar entropy of H2O(1) is 69.91 J.mol-1 K-1. Calculate the standard molar entropy of H2O(g).
Step by Step Solution
There are 3 Steps involved in it
Step: 1
33 To calculate the change in specific entropy ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
4th edition
978-1118431221, 9781119192138, 1118431227, 1119192137, 978-1119498759
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



