Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. 5. 6. Using your answers from questions 4 and 5( the 2 answers from above that I showed u ), create a bar graph
4.
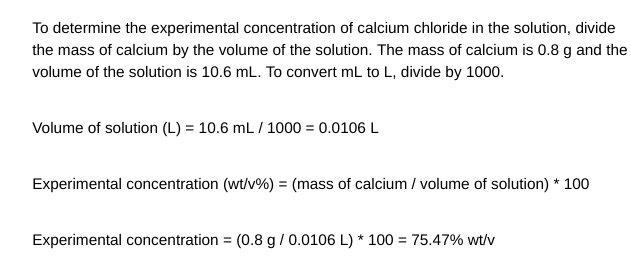
5.
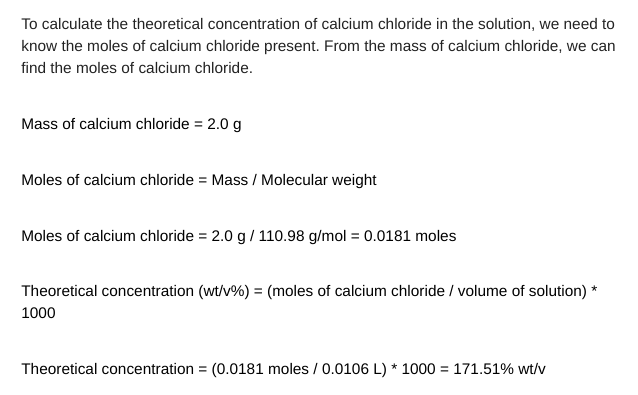
6. Using your answers from questions 4 and 5( the 2 answers from above that I showed u ), create a bar graph comparing the theoretical to experimental percent concentrations of calcium chloride in the solution. Then, calculate the percent error between the two concentrations.
To determine the experimental concentration of calcium chloride in the solution, divide the mass of calcium by the volume of the solution. The mass of calcium is 0.8g and the volume of the solution is 10.6mL. To convert mL to L, divide by 1000 . Volume of solution (L)=10.6mL/1000=0.0106L Experimental concentration (wt/v%)=( mass of calcium / volume of solution) 100 Experimental concentration =(0.8g/0.0106L)100=75.47%wt/v To calculate the theoretical concentration of calcium chloride in the solution, we need to know the moles of calcium chloride present. From the mass of calcium chloride, we can find the moles of calcium chloride. Mass of calcium chloride =2.0g Moles of calcium chloride = Mass / Molecular weight Moles of calcium chloride =2.0g/110.98g/mol=0.0181 moles Theoretical concentration (wt/v%)=( moles of calcium chloride / volume of solution ) * 1000 Theoretical concentration =(0.0181 moles /0.0106L)1000=171.51%wt/vStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


