Answered step by step
Verified Expert Solution
Question
1 Approved Answer
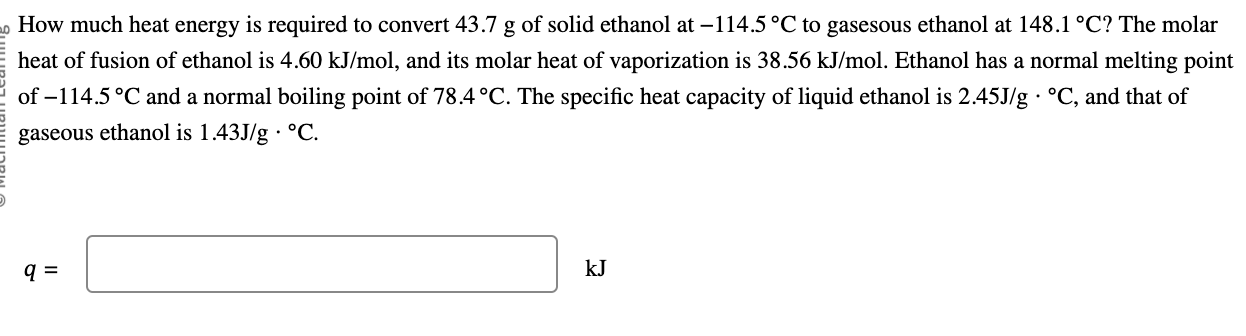
How much heat energy is required to convert 43.7 g of solid ethanol at 114.5C to gasesous ethanol at 148.1C ? The molar heat of
How much heat energy is required to convert 43.7 g of solid ethanol at 114.5C to gasesous ethanol at 148.1C ? The molar heat of fusion of ethanol is 4.60 kJ/mol , and its molar heat of vaporization is 38.56 kJ/mol . Ethanol has a normal melting point of 114.5C and a normal boiling point of 78.4C . The specific heat capacity of liquid ethanol is 2.45J/gC , and that of gaseous ethanol is 1.43J/gC .
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


