Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. 5. In which of following conditions a real gas would behave ideally? (a) Low pressure and low temperature ha (b) At value of
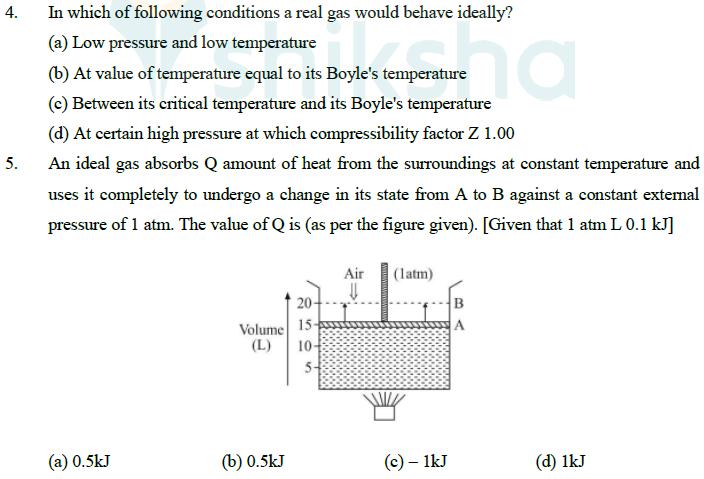
4. 5. In which of following conditions a real gas would behave ideally? (a) Low pressure and low temperature ha (b) At value of temperature equal to its Boyle's temperature (c) Between its critical temperature and its Boyle's temperature (d) At certain high pressure at which compressibility factor Z 1.00 An ideal gas absorbs Q amount of heat from the surroundings at constant temperature and uses it completely to undergo a change in its state from A to B against a constant external pressure of 1 atm. The value of Q is (as per the figure given). [Given that 1 atm L 0.1 kJ] Air (latm) U 20- Volume 15- (L) 10- 505 5+ B (a) 0.5kJ (b) 0.5kJ (c) - 1kJ (d) 1kJ
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


