Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. A tube of 1m length is open at one end and is filled with a stoichiometric CH4/ air mixture. The temperature distribution in the

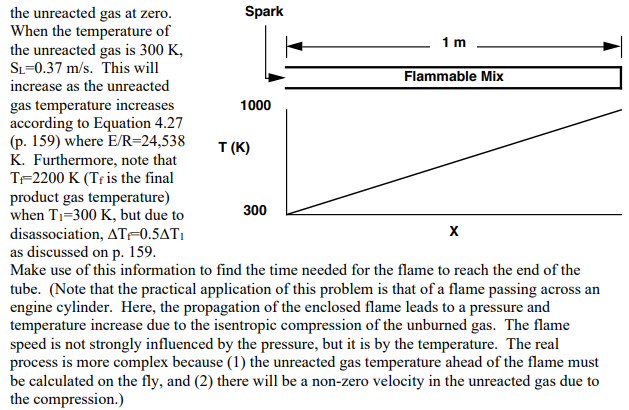 4. A tube of 1m length is open at one end and is filled with a stoichiometric CH4/ air mixture. The temperature distribution in the unreacted gas (T1) is as shown in the figure. At time zero the mixture is sparked at the open end and a flame propagates down the tube. The open end allows the pressure to stay at one atmosphere and keeps the laboratory coordinate velocity of the unreacted gas at zero. When the temperature of the unreacted gas is 300K, SL=0.37m/s. This will increase as the unreacted gas temperature increases according to Equation 4.27 (p. 159) where E/R=24,538 K. Furthermore, note that Tf=2200K ( Tf is the final product gas temperature) when T1=300K, but due to disassociation, Tf=0.5T1 as discussed on p. 159 . Make use of this information to find the time needed for the flame to reach the end of the tube. (Note that the practical application of this problem is that of a flame passing across an engine cylinder. Here, the propagation of the enclosed flame leads to a pressure and temperature increase due to the isentropic compression of the unburned gas. The flame speed is not strongly influenced by the pressure, but it is by the temperature. The real process is more complex because (1) the unreacted gas temperature ahead of the flame must be calculated on the fly, and (2) there will be a non-zero velocity in the unreacted gas due to the compression.)
4. A tube of 1m length is open at one end and is filled with a stoichiometric CH4/ air mixture. The temperature distribution in the unreacted gas (T1) is as shown in the figure. At time zero the mixture is sparked at the open end and a flame propagates down the tube. The open end allows the pressure to stay at one atmosphere and keeps the laboratory coordinate velocity of the unreacted gas at zero. When the temperature of the unreacted gas is 300K, SL=0.37m/s. This will increase as the unreacted gas temperature increases according to Equation 4.27 (p. 159) where E/R=24,538 K. Furthermore, note that Tf=2200K ( Tf is the final product gas temperature) when T1=300K, but due to disassociation, Tf=0.5T1 as discussed on p. 159 . Make use of this information to find the time needed for the flame to reach the end of the tube. (Note that the practical application of this problem is that of a flame passing across an engine cylinder. Here, the propagation of the enclosed flame leads to a pressure and temperature increase due to the isentropic compression of the unburned gas. The flame speed is not strongly influenced by the pressure, but it is by the temperature. The real process is more complex because (1) the unreacted gas temperature ahead of the flame must be calculated on the fly, and (2) there will be a non-zero velocity in the unreacted gas due to the compression.) Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


