Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. An ideal gas undergoes a thermodynamic process in which internal energy aP4, where a is a (U) of the gas depends on pressure

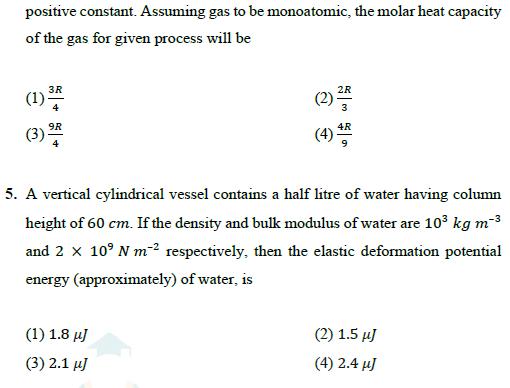
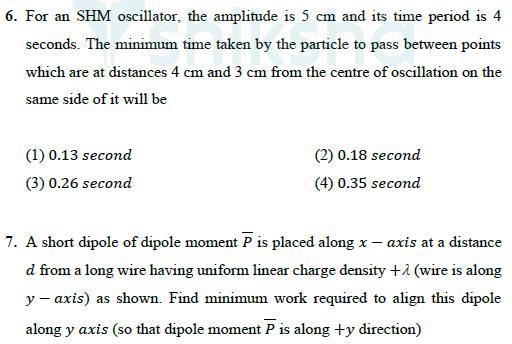
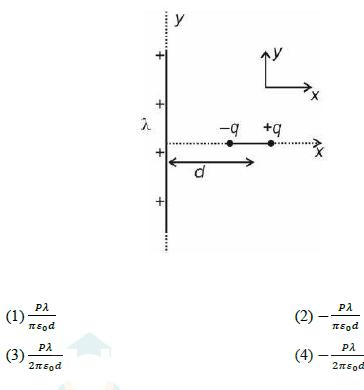
4. An ideal gas undergoes a thermodynamic process in which internal energy aP4, where a is a (U) of the gas depends on pressure (P) of the gas as = positive constant. Assuming gas to be monoatomic, the molar heat capacity of the gas for given process will be 3R (1) 34 9R (3) 94 4 2R (2) 21 4R (4) 5. A vertical cylindrical vessel contains a half litre of water having column height of 60 cm. If the density and bulk modulus of water are 103 kg m-3 and 2 109 N m respectively, then the elastic deformation potential energy (approximately) of water, is (1) 1.8 J (3) 2.1 J (2) 1.5 J (4) 2.4 J 6. For an SHM oscillator, the amplitude is 5 cm and its time period is 4 seconds. The minimum time taken by the particle to pass between points which are at distances 4 cm and 3 cm from the centre of oscillation on the same side of it will be (1) 0.13 second (3) 0.26 second (2) 0.18 second (4) 0.35 second 7. A short dipole of dipole moment P is placed along x-axis at a distance d from a long wire having uniform linear charge density +1 (wire is along y-axis) as shown. Find minimum work required to align this dipole along y axis (so that dipole moment P is along +y direction) + y + d -q +q (1) (3) P 2sod (2)- P 2so (4)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


