Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. Calculate pH from the following hydrogen ion concentration (M). Identify each as an acidic pH or a basic pH. pH Basic/Acidic Hydrogen lon Concentration
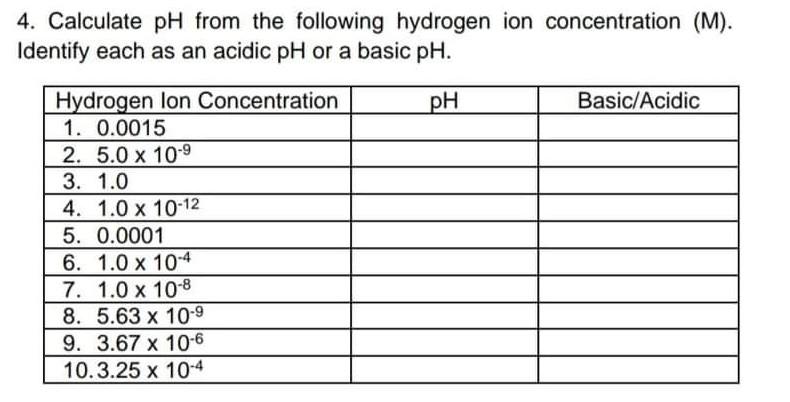

4. Calculate pH from the following hydrogen ion concentration (M). Identify each as an acidic pH or a basic pH. pH Basic/Acidic Hydrogen lon Concentration 1. 0.0015 2. 5.0 x 10-9 3. 1.0 4. 1.0 x 10-12 5. 0.0001 6. 1.0 x 10-4 7. 1.0 x 10-8 8. 5.63 x 10-9 9. 3.67 x 10-6 10.3.25 x 10-4 Activity 2: BALANCING REDOX REACTIONS Directions: Inspect the given reactions very carefully, follow the given step- by-step procedure to balance the equation. 1. 12(g) + Cl2(g) + H2O HIO3(aq) + HCl(aq) 2. Sn(s) + HNO3(aq) SnO2(s) + NO2() + H2O) 3. Al(s) + Ag(aq) Al3+ + Ag(s) 4. Kis) + Cr3+ (aq) Cris) + K+ (aq) 5. Zn(s) + Sn2+ (aq) Zn2+ (aq) + Sn(s)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


