Answered step by step
Verified Expert Solution
Question
1 Approved Answer
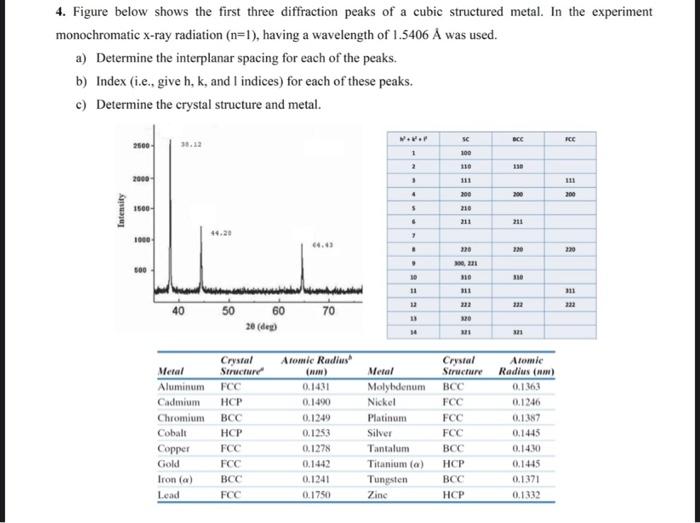
4. Figure below shows the first three diffraction peaks of a cubic structured metal. In the experiment monochromatic x-ray radiation (n=1), having a wavelength of
4. Figure below shows the first three diffraction peaks of a cubic structured metal. In the experiment monochromatic x-ray radiation (n=1), having a wavelength of 1.5406 was used. a) Determine the interplanar spacing for each of the peaks. b) Index (i.e., give h, k, and I indices) for each of these peaks. c) Determine the crystal structure and metal. Intensity 2500- 2000- 1500- 1000- 500 38.12 40 44.29 Cobalt Copper Gold Iron (a) Lead 50 Metal Aluminum FCC Cadmium HCP Chromium BCC HCP FCC FCC BCC FCC Crystal Structure 60 20 (deg) 64.43 70 Atomic Radius (nm) 0.1431 0.1490 0.1249 0.1253 0.1278 0.1442 0.1241 0.1750 Metal W+K+F 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Molybdenum Nickel Platinum Silver Tantalum Titanium (a) Tungsten Zinc SC 100 110 111 200 210 211 220 300, 221 310 311 222 320 321 BCC BCC FCC FCC FCC BCC HCP BCC HCP 110 200 211 220 310 222 321 Crystal Atomic Structure Radius (nm) 0.1363 0.1246 0.1387 0.1445 0.1430 0.1445 0.1371 0.1332 FCC 111 200 220 311 222
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


