Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4) For the 2-propanol (1)-water (2) system, the following Wilson parameters are reported. a12=1833.74J/molV1=76.92106m3/mol,a21=5183.26J/molV2=18.07106m3/mol, The vapour pressures can be calculated by the Antoine equations, which
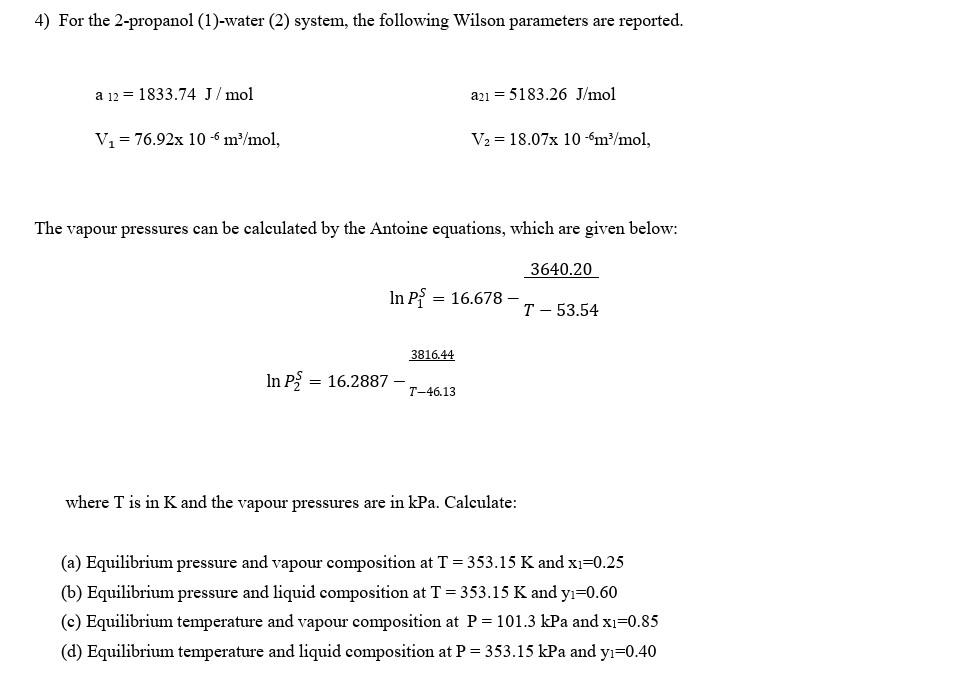
4) For the 2-propanol (1)-water (2) system, the following Wilson parameters are reported. a12=1833.74J/molV1=76.92106m3/mol,a21=5183.26J/molV2=18.07106m3/mol, The vapour pressures can be calculated by the Antoine equations, which are given below: lnP1S=16.678T53.543640.20lnP2S=16.2887T46.13 3816.44 where T is in K and the vapour pressures are in kPa. Calculate: (a) Equilibrium pressure and vapour composition at T=353.15K and x1=0.25 (b) Equilibrium pressure and liquid composition at T=353.15K and y1=0.60 (c) Equilibrium temperature and vapour composition at P=101.3kPa and x1=0.85 (d) Equilibrium temperature and liquid composition at P=353.15kPa and y1=0.40
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


