Question
4. Ozone decomposes according to the following reaction: 2 03 (g) 3 02 (g) a) Use the table below to calculate AG,rxn at 230
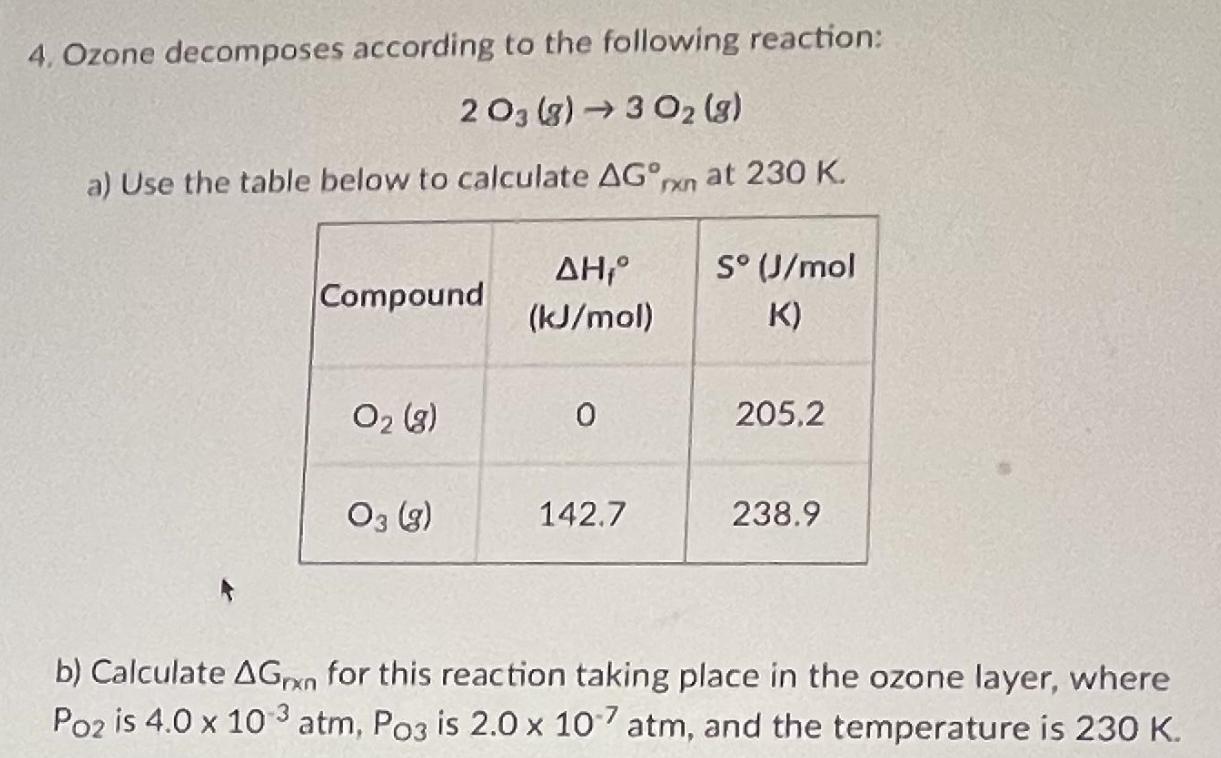
4. Ozone decomposes according to the following reaction: 2 03 (g) 3 02 (g) a) Use the table below to calculate AG,rxn at 230 K. S (J/mol Compound (kJ/mol) K) O2 (g) 205.2 O3 (g) 142.7 238.9 b) Calculate AGXO for this reaction taking place in the ozone layer, where Po2 is 4.0 x 10 3 atm, Po3 is 2.0 x 10 atm, and the temperature is 230 K.
Step by Step Solution
3.50 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Sol The free energy change of the reaction in any state G when equilibr...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
International Economics
Authors: James Gerber
6th edition
978-0132950145, 132950146, 132948915, 978-0132948913
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



