Question
4. Suppose that n moles of an ideal gas initially is confined within a volume V and held at temperature T. The gas is
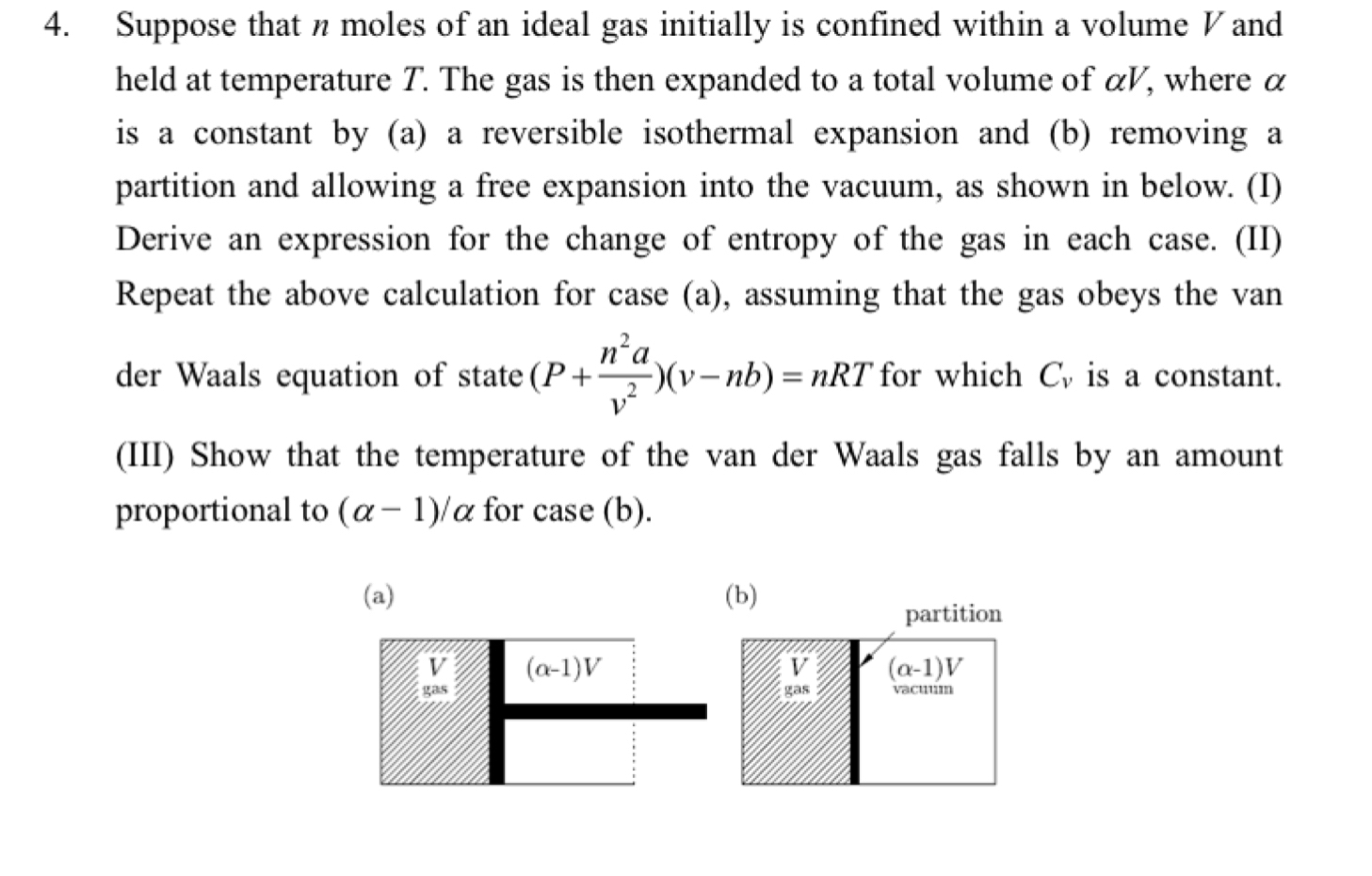
4. Suppose that n moles of an ideal gas initially is confined within a volume V and held at temperature T. The gas is then expanded to a total volume of aV, where a is a constant by (a) a reversible isothermal expansion and (b) removing a partition and allowing a free expansion into the vacuum, as shown in below. (I) Derive an expression for the change of entropy of the gas in each case. (II) Repeat the above calculation for case (a), assuming that the gas obeys the van na. der Waals equation of state (P+ -)(v-nb) = nRT for which C, is a constant. 2 (III) Show that the temperature of the van der Waals gas falls by an amount proportional to (a- 1)/a for case (b). (a) gas (a-1)V (b) gas partition (a-1)V vacuum
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Chemical Engineering Thermodynamics
Authors: Kevin D. Dahm, Donald P. Visco
1st Edition
1111580707, 978-1111580704
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



