Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(4). The disaccharide lactose can be decomposed into its constituent sugars galactose and glucose. This decomposition can be accomplished through acid-based hydrolysis or by the

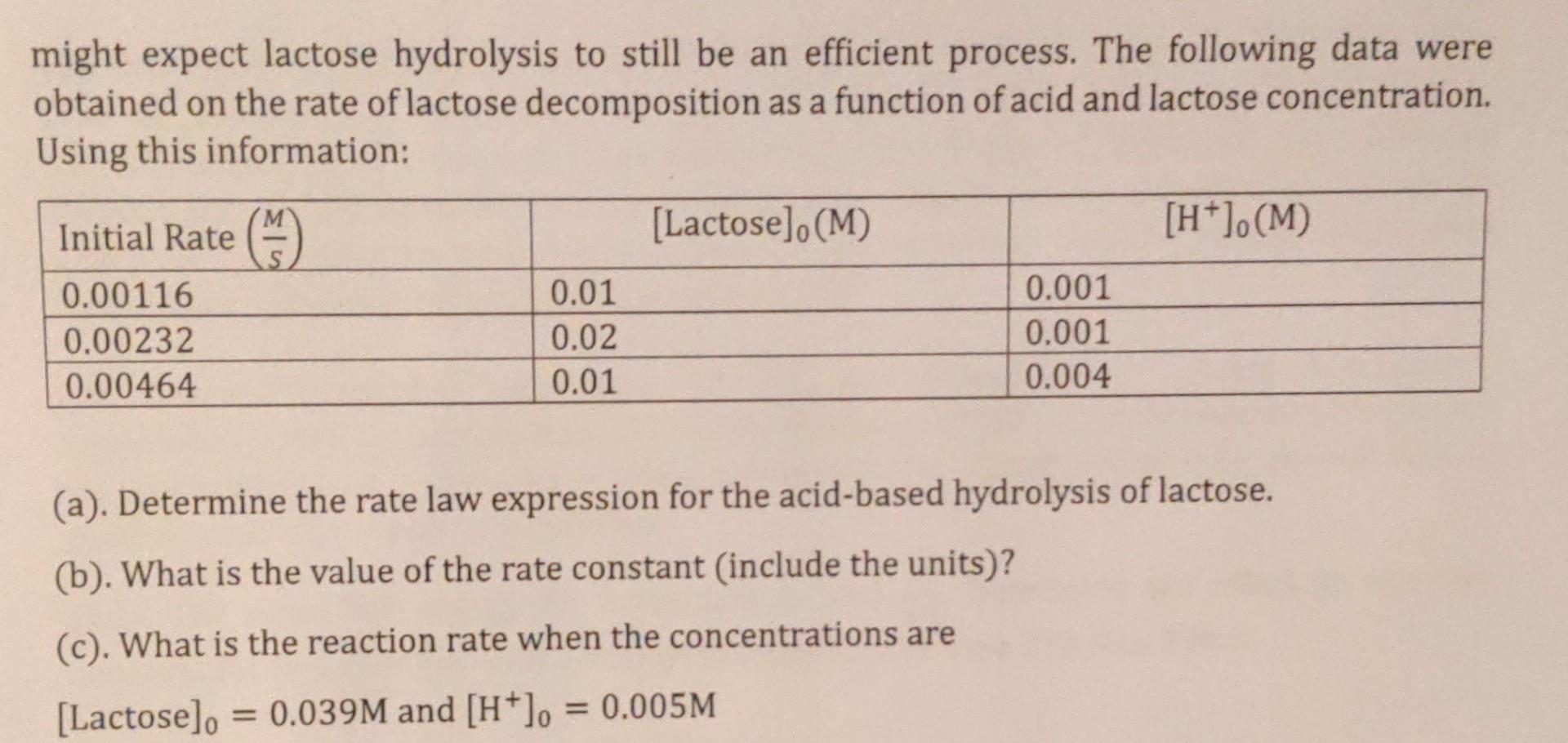
(4). The disaccharide lactose can be decomposed into its constituent sugars galactose and glucose. This decomposition can be accomplished through acid-based hydrolysis or by the enzyme lactase. Lactose intolerance in humans is due to the lack of lactase production by cells in the small intestine. However, the stomach is an acidic environment; therefore, one might expect lactose hydrolysis to still be an efficient process. The following data were obtained on the rate of lactose decomposition as a function of acid and lactose concentration. Using this information: (a). Determine the rate law expression for the acid-based hydrolysis of lactose. (b). What is the value of the rate constant (include the units)? (c). What is the reaction rate when the concentrations are [Lactose]0=0.039M and [H+]0=0.005M
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


