Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gas mixture of methane and steam at atmospheric pressure and 500C is fed to a reactor, where the following reactions occur: CH4 +
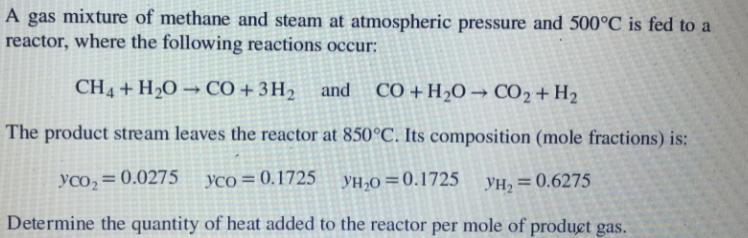
A gas mixture of methane and steam at atmospheric pressure and 500C is fed to a reactor, where the following reactions occur: CH4 + H20 CO + 3H2 and C +H2O CO2 + H2 The product stream leaves the reactor at 850C. Its composition (mole fractions) is: yco, = 0.0275 yco=0.1725 VH,0=0.1725 YH, =0.6275 Determine the quantity of heat added to the reactor per mole of produet gas.
Step by Step Solution
★★★★★
3.31 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
609a110b7a8a0_212751.pdf
180 KBs PDF File
609a110b7a8a0_212751.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


